Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
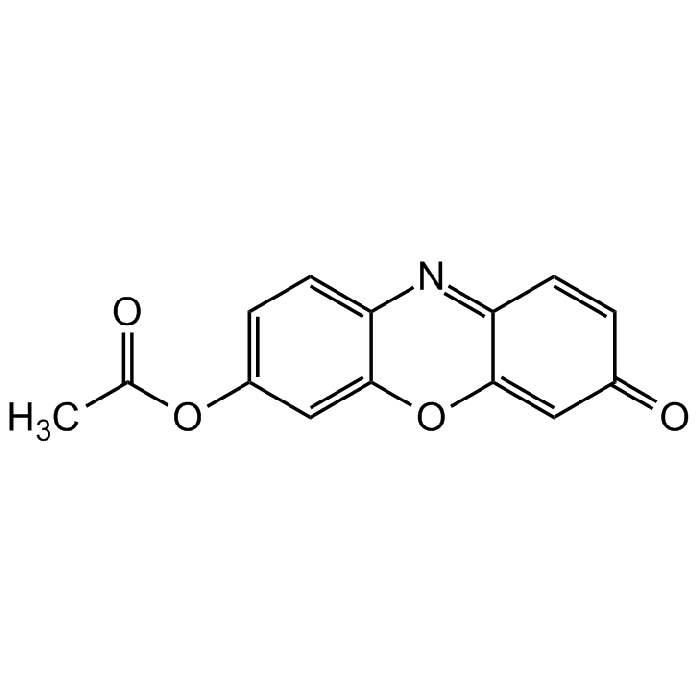
Resorufin acetate
| Product Details | |
|---|---|
| Synonyms | O-acetyl Resorufin; 7-Acetyl-3H-phenoxazin-3-one |
| Product Type | Chemical |
| Properties | |
| Formula | C14H9NO4 |
| MW | 255.23 |
| CAS | 1152-14-3 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥98% (TLC) |
| Appearance | Orange to dark orange powder. |
| Solubility | Soluble in DMSO (5mg/ml) or DMF (20mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | UJWKHDKBOVPINX-UHFFFAOYSA-N |
| Smiles | O=C1C=C2OC3=CC(OC(C)=O)=CC=C3N=C2C=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Resorufin acetate is a fluorogenic substrate for hydrolytic esterases and cellulases used for monitoring cytosolic aldehyde dehydrogenase (ALDH1A1) esterase activity, chymotrypsin activity. Resorufin acetate has been used as a novel indicator reaction for fluorometric detection of glucose using only glucose oxidase (GOD), without significant effects of ascorbic acid, uric acid, or bilirubin. It also has been used for the chromogenic and fluorescent detection of hydrazine, acyl protein thioesterase (APT) enzymes and perborate ions, and reveals a selective turn-on type chromogenic and fluorogenic signaling behavior based on the selective and efficient cleavage of acetate group. Upon enzymatic cleavage of the acetate group of resorufin acetate, the fluorescent hydrolysis product resorufin is released and its fluorescence can be used to quantify enzyme activity. Spectral data of Resorufin: λex=571nm, λem=585nm.
(1) G.G. Guilbault & A.N.J. Heyn; Anal. Lett. 1, 163 (1967) | (2) T.M. Kitson & K.E. Kitson; Biochem J. 322, 701 (1997) | (3) T.M. Kitson & K.E. Kitson; Adv. Exp. Med. Biol. 414, 201 (1997) | (4) T.M. Kitson; Biochim. Biophys. Acta 1385, 43 (1998) | (5) H. Maeda, et al.; Chem. Pharm. Bull. 49, 294 (2001) | (6) M.G. Choi, et al.; Org. Lett. 12, 1468 (2010) | (7) M.G. Choi, et al.; Org. Biomol. Chem. 11, 2961 (2013)





