Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
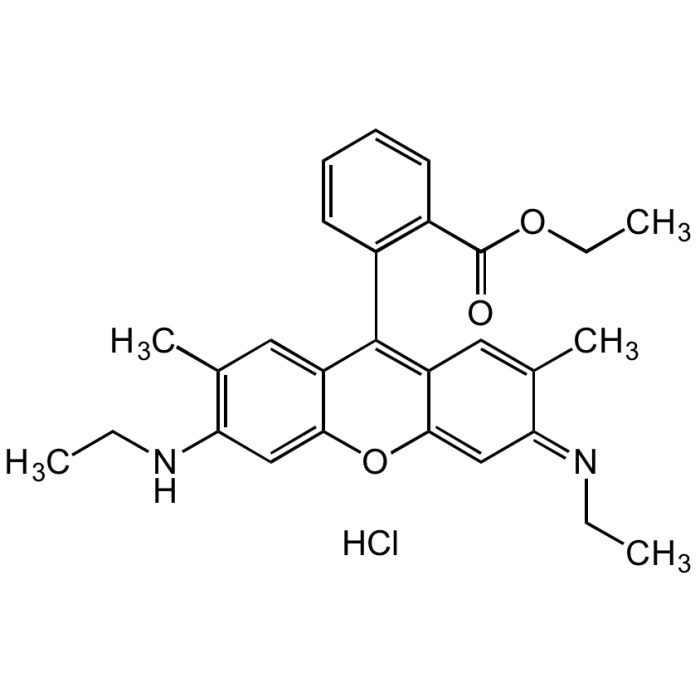
Rhodamine 6G
| Product Details | |
|---|---|
| Synonyms | 6-Carboxyrhodamine; Aizen Rhodamine 6GCP; Basic Red 1; C.I. 45160; Calcozine Red 6G; NSC 36345 |
| Product Type | Chemical |
| Properties | |
| Formula |
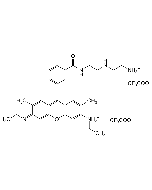
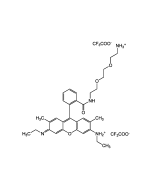
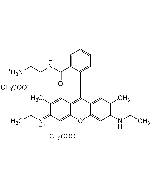
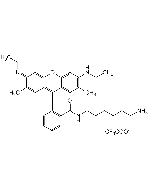
C28H30N2O3 . HCl |
| MW | 442.5 . 36.5 |
| CAS | 989-38-8 |
| Source/Host Chemicals | Synthetic |
| Appearance | Greenish red to brown powder. |
| Solubility | Soluble in methanol, water or ethanol. |
| Identity | Determined by NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | VYXSBFYARXAAKO-WTKGSRSZSA-N |
| Smiles | Cl.CCNC1=C(C)C=C2C(OC3=C\C(=N/CC)C(C)=CC3=C2C2=C(C=CC=C2)C(=O)OCC)=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Handling Advice |
Keep cool and dry. Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Rhodamine 6G is a highly fluorescent hydrophilic pH-sensitive dye. It is often used as a tracer dye within water to determine the rate and direction of flow and transport. Rhodamine dyes are used extensively in biotechnology applications such as fluorescence microscopy, flow cytometry, fluorescence correlation spectroscopy and ELISA. The dye has a remarkably high photostability and high fluorescence quantum yield. Used as a laser dye and potential mitochondrial probe. The lasing range of the dye is 555 to 585 nm with a maximum at 566 nm. Useful in Pgp efflux assays and has been used to characterize kinetics of MRP1-mediated efflux. Spectral data: λex 528 nm| λem 551 nm (in MeOH).
(1) T. Aiuchi, et al.; Biochim. Biophys. Acta. 685, 289 (1982) | (2) R.F. Kubin & A.N. Fletcher; J. Luminesc. 27, 455 (1983) | (3) M.W. Berns, et al.; Cell Biophys. 6, 263 (1984) | (4) Y. Matsumoto, et al.; J. Neurooncol. 13, 217 (1992) | (5) M. Mandala, et al.; Anal. Biochem. 274, 1 (1999) | (6) D. Magde, et al.; Photochem. Photobiol. 75, 327 (2002) | (7) Z. Chen, et al.; J. Fluoresc. 18, 93 (2008) | (8) T. Baumgartel, et al.; Nanotechnology 21, 475205 (2010)