Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
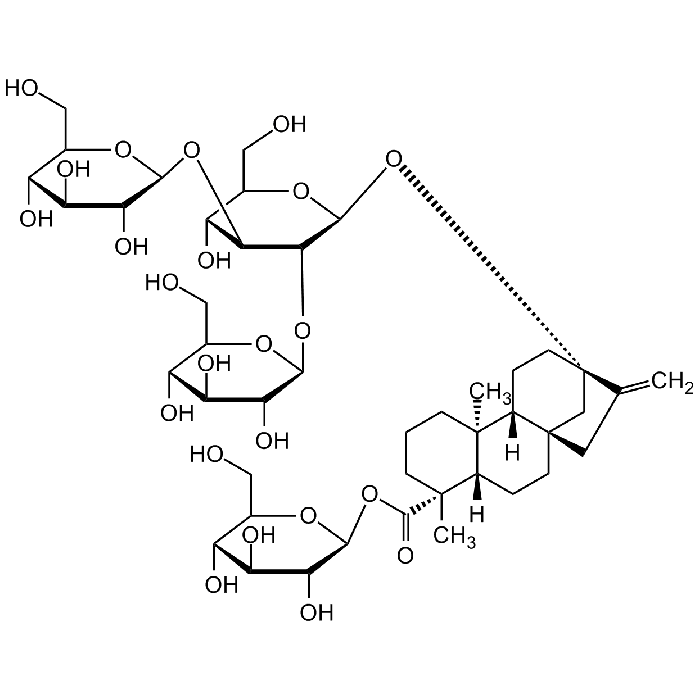
Rebaudioside A
| Product Details | |
|---|---|
| Synonyms | Glycoside A3; Glycoside X; Stevioside A3; Reb A |
| Product Type | Chemical |
| Properties | |
| Formula |
C44H70O23 |
| MW | 967.01 |
| CAS | 58543-16-1 |
| Source/Host Chemicals | Plant |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White powder. |
| Solubility | Soluble in water (10mg/ml) or DMSO. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | HELXLJCILKEWJH-NCGAPWICSA-N |
| Smiles | C=C1[C@@]2(O[C@H]3[C@H](O[C@H]4[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O4)[C@@H](O[C@H]5[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O5)[C@H](O)[C@@H](CO)O3)C[C@]6(C1)CC[C@]7([H])[C@](C)(C(O[C@H]8[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O8)=O)CCC[C@@]7(C)[C@]6([H])CC2 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at RT. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Rebaudioside A is a glucosylated steviol glycoside studied and used as a non-glycemic sweetener. It is one of the predominant steviol glycosides isolated from S. rebaudiana leaves. Rebaudioside A is a α-glucosidase inhibitor with (IC50=35μg/ml) and can inhibit ATP-sensitive K+-channels. In vitro rebaudioside A stimulated the insulin secretion from MIN6 cells in a dose- and glucose-dependent manner. It increases glucagon-like peptide 1 (GLP-1) secretion in a 2-dimensional mouse intestine model. Rebaudioside A is metabolized by gut microbiota to steviol. Rebaudioside A shows antioxidant activity on reducing cellular reactive oxygen species. It is an activator of Nrf2 and is a potential candidate hepatoprotective agent.
(1) R. Abudula, et al.; Metab.53, 1378 (2004) | (2) R. Abudula, et al.; Diabetes Obes. Metab. 10, 1074 (2008) | (3) D.J. Brusick; Food Chem. Toxicol. 46, S83 (2008) | (4) M. Upreti, et al.; Molecules 17, 4186 (2012) | (5) D. Ripken, et al.; J. Agric. Food Chem. 62, 8365 (2014) | (6) N. van der Wielen, et al.; J. Nutr. 146, 2429 (2016) | (7) D.H. Shin, et al.; Diabetes Metab. J. 40, 283 (2016) | (8) B.R. Adari, et al.; Food Chem. 200, 154 (2016) | (9) Y. Wang, et al.; Eur. J. Pharmacol. 822, 128 (2018)





