Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
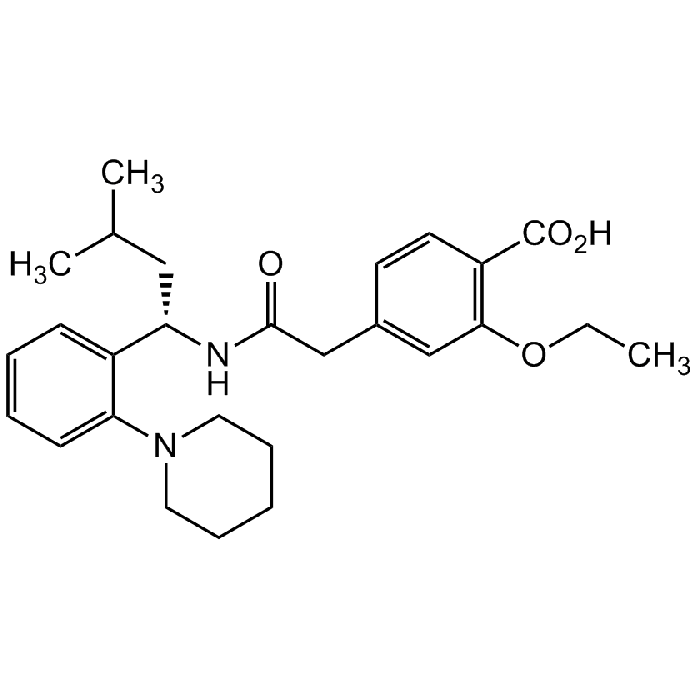
Repaglinide
| Product Details | |
|---|---|
| Synonyms | (S)-(+)-2-Ethoxy-4-[N-[1-(2-piperidinophenyl)-3-methyl-1-butyl]aminocarbonylmethyl]benzoic acid; Novonorm; Prandin |
| Product Type | Chemical |
| Properties | |
| Formula |
C27H36N2O4 |
| MW | 452.59 |
| CAS | 135062-02-1 |
| RTECS | DI0876305 |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in DMSO (30mg/ml), DMF (30mg/ml) or ethanol (20mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | FAEKWTJYAYMJKF-QHCPKHFHSA-N |
| Smiles | O=C(CC1=CC(OCC)=C(C(O)=O)C=C1)N[C@@H](CC(C)C)C2=CC=CC=C2N3CCCCC3 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Repaglinide is a metaglitinide antidiabetic agent. It is a potent short-acting insulin secretagogue that acts by closing ATP-sensitive potassium (KATP) channels in the plasma membrane of the pancreatic beta cell. It represents a new class of insulin secretagogues, structurally unrelated to sulphonylureas, which were developed for the treatment of type 2 diabetes. Repaglinide shows also antioxidant and anti-inflammatory properties.
(1) B.H. Wolffenbuttel, et al.; Eur. J. Clin. Pharmacol. 45, 113 (1993) | (2) J. Gromada, et al.; Diabetologia 38, 1025 (1995) | (3) D.R. Owens; Eur. J. Clin. Invest. 29, 30 (1999) (Review) | (4) V. Ambavane, et al.; J. Postgrad. Med. 48, 246 (2002) (Review) | (5) A.M. Hansen, et al.; Br. J. Pharmacol. 144, 551 (2005) | (6) A. Gumieniczek, et al.; Pharmacol. Res. 52, 162 (2005) | (7) D. Tung, et al.; Pharmacology 88, 295 (2011) | (8) P. Kuhner, et al.; Naunyn Schmiedebergs Arch. Pharmacol. 385, 299 (2012) | (9) L.J. Scott; Drugs 72, 249 (2012) (Review) | (10) D. Ding, et al.; Cell Rep. 27, 1848 (2019)





