Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
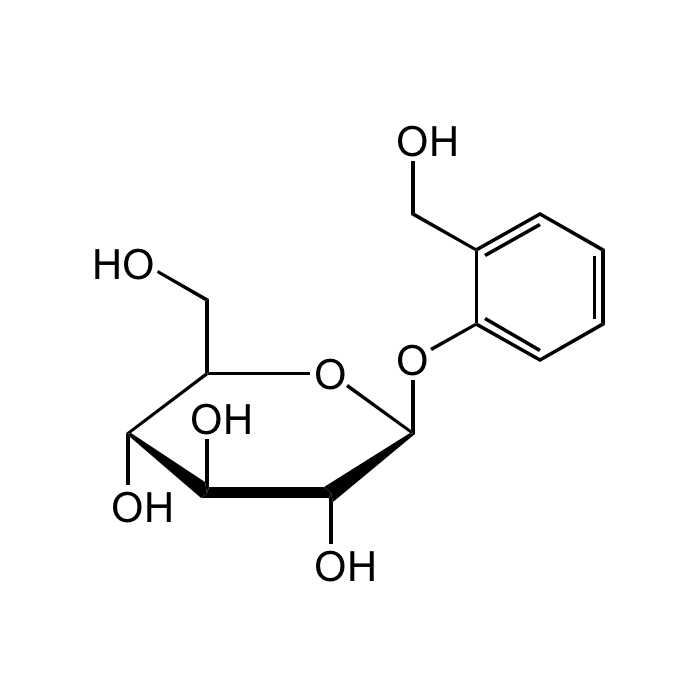
Salicin
| Product Details | |
|---|---|
| Synonyms | D-(-)-Salicin; 2-(Hydroxymethyl)phenyl-β-D-glucopyranoside; Salicoside; Salicyl alcohol glucoside; Saligenin β-D-glucoside; NSC 5751 |
| Product Type | Chemical |
| Properties | |
| Formula |
C13H18O7 |
| MW | 286.28 |
| CAS | 138-52-3 |
| RTECS | LZ5901700 |
| Source/Host Chemicals | Isolated from plant source. |
| Purity Chemicals | ≥99% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in water (10mg/ml), DMSO (20mg/ml) or DMF (30mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | NGFMICBWJRZIBI-UJPOAAIJSA-N |
| Smiles | OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@H](OC2=CC=CC=C2CO)O1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at RT. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Salicin is an alcoholic β-glucoside derived from willow bark that produces antioxidant and anti-inflammatory (including analgesic and antipyretic) effects very similar to that of aspirin. It acts as a non-selective COX inhibitor for COX-1 and COX-2. It has been used in the treatment of rheumatism. Shown to inhibit angiogenesis by blocking the ROS-ERK pathways and to have in vivo anticancer activity.
(1) T. Maclagan; Br. Med. J. 1, 627 (1876) | (2) T.D. Warner, et al.; PNAS 96, 7563 (1999) | (3) I. Wagner & L. Heide; J. Rheumatol. 30, 1125 (2003) | (4) A.L. Blobaum & L.J. Marnett; J. Med. Chem. 50, 1425 (2007) | (5) N. Verma, et al.; Inflamm. Res. 63, 161 (2014) | (6) C.S. Kong, et al.; Phytother. Res. 28, 1246 (2014) | (7) Y. Li, et al.; Int. Immunopharmacol. 26, 286 (2015) | (8) M. Sabaa, et al.; Naunyn Schmiedebergs Arch. Pharmacol. 390, 1061 (2017)





