Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
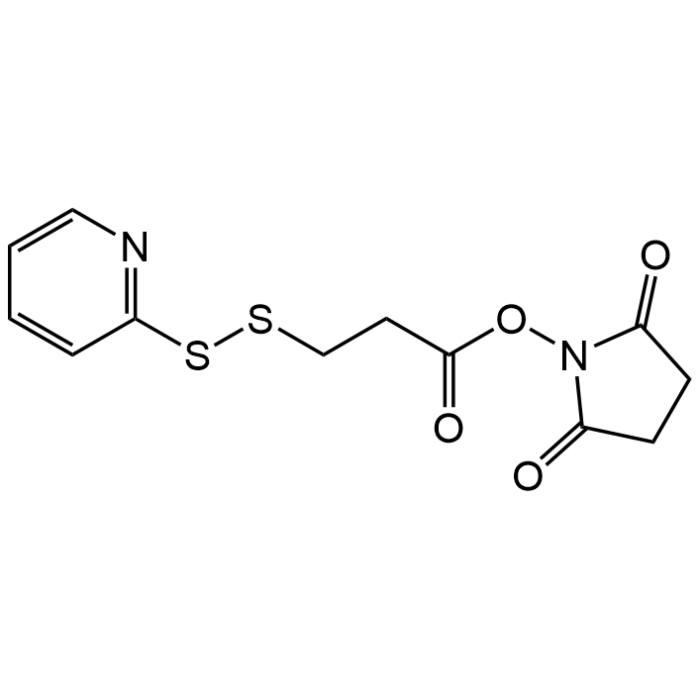
N-Succinimidyl 3-(2-pyridyldithio)propionate
| Product Details | |
|---|---|
| Synonyms | 3-(2-Pyridyldithio)propionic acid N-hydroxysuccinimide ester; SPDP |
| Product Type | Chemical |
| Properties | |
| Formula | C12H12N2O4S2 |
| MW | 312.36 |
| CAS | 68181-17-9 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥97% (NMR) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in acetone (50mg/ml), DMF or methanol. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | JWDFQMWEFLOOED-UHFFFAOYSA-N |
| Smiles | O=C(CCSSC1=NC=CC=C1)ON2C(CCC2=O)=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
N-Succinimidyl 3-(2-pyridyldithio)propionate is a heterobifunctional cross-linking reagent with amine and sulfhydryl reactivity. It is typically coupled initially to molecules containing primary amines by amide bonds buffered at pH 7.5 (6.5-8.5). Second coupling specific for molecules containing free sulfhydryl by thiol-disulfide exchange buffered at pH 8.0 (7.5-8.5). Alternately, this reagent may be used as a protected thiolating reagent following initial coupling and reduction of linker. It incorporates four atom linker. N-Succinimidyl 3-(2-pyridyldithio)propionate (SPDP) is useful for the preparation of enzyme immunoconjugates and hapten carrier molecule conjugates. It has been used for antibody conjugation and as a protein cross-linker. It is also a glutathione cleavable ADC linker used for the antibody-drug conjugates (ADCs).
(1) J. Carlsson, et al.; Biochem. J. 173, 723 (1978) | (2) D. Pain & A. Surolia; J. Immunol. Methods 40, 219 (1981) | (3) P. Nilsson, et al.; J. Immunol. Methods 41, 81 (1981) | (4) A.F. Habeeb; Biochim. Biophys. Acta 673, 527 (1981) | (5) Y.H. Jou, et al.; Immunol. Commun. 11, 357 (1982) | (6) Y.H. Jou, et al.; Methods Enzymol. 92, 257 (1983)





