Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
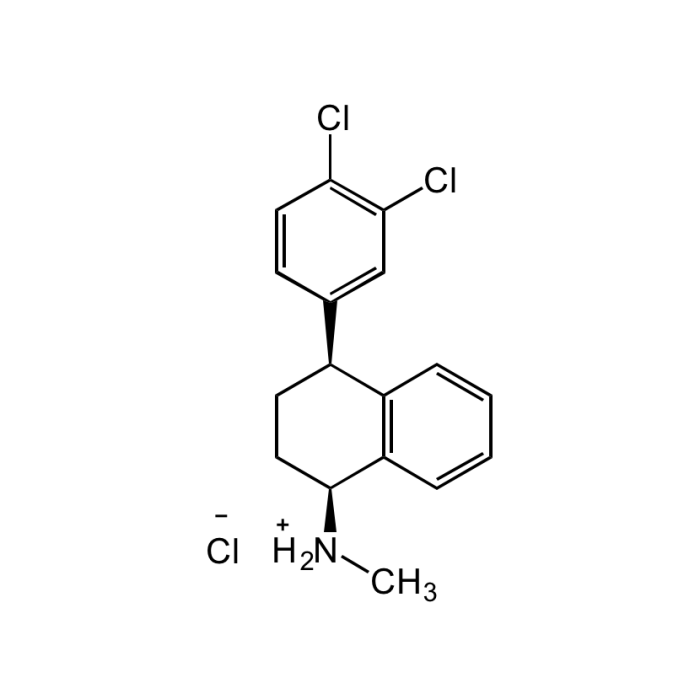
Sertraline hydrochloride
| Product Details | |
|---|---|
| Synonyms | (1S,4S)-4-(3,4-Dichlorophenyl)-1,2,3,4-tetrahydro-N-methyl-1-naphthalenamine hydrochloride; CP 51,974-1; Lustral; Seserine; Zoloft |
| Product Type | Chemical |
| Properties | |
| Formula |
C17H17NCl2 . HCl |
| MW | 342.69 |
| CAS | 79559-97-0 |
| RTECS | QJ0352070 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in DMSO (20mg/ml) or ethanol (10mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | BLFQGGGGFNSJKA-XHXSRVRCSA-N |
| Smiles | ClC(C=C1)=C(Cl)C=C1[C@H]2C3=C(C=CC=C3)[C@@H]([NH2+]C)CC2.[Cl-] |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | -20°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Sertraline is a tetraline that inhibits monoamine transporters and acts as a potent highly selective serotonin reuptake inhibitor (SSRI), with affinity for the serotonin transporter (SERT), increasing availability of serotonin (5-hydroxytryptamine; 5-HT) in the brain and leading to antidepressant, anxiolytic and antiobsessional effects. Sertraline binds also to the dopamine transporter (DAT) and the σ1 receptor (but not the σ2 receptor) with 100-fold lower affinity. It acts as an antagonist of the σ1 receptor, and is able to reverse σ1 receptor-dependent actions of fluvoxamine, a potent agonist of the receptor, in vitro. Sertraline displays antiproliferative activity on human colorectal carcinoma cells.
(1) B.K. Koe, et al.; J. Pharmacol. Exp. Ther. 226, 686 (1983) | (2) W.M. Welch, et al.; J. Med. Chem. 27, 1508 (1984) | (3) J. Heym & B.K. Koe; J. Clin. Psychiatry 49, 40 (1988) | (4) M. Tatsumi, et al.; Eur. J. Pharmacol. 340, 249 (1997) | (5) G. MacQueen, et al.; CNS Drug Reviews 7, 1 (2001) | (6) H.R. Khouzam, et al.; Compr. Ther. 29, 47 (2003) | (7) J.H. Meyer, et al.; Am. J. Psych. 161, 826 (2004) | (8) B. Lowe, et al.; J. Affect. Disord. 87, 271 (2005) | (9) Y. Albayrak & K. Hashimoto; Adv. Exp. Med. Biol. 964, 153 (2017)





