Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
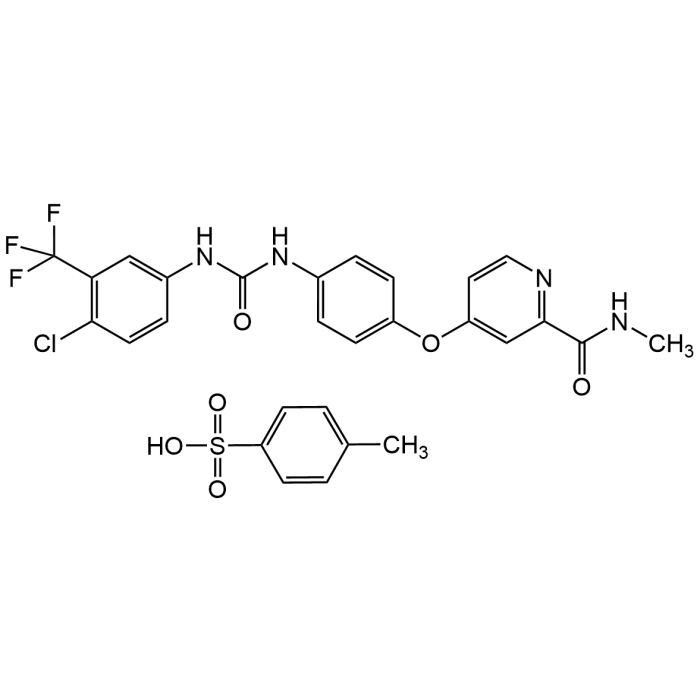
Sorafenib tosylate
As low as
77
CHF
CHF 77.00
In stock
Only %1 left
CDX-S0264-G0055 gCHF 77.00
CDX-S0264-G02525 gCHF 309.00
| Product Details | |
|---|---|
| Synonyms | Sorafenib p-toluenesulfonate; Nexavar; ,BAY 43-9006 mono-p-tosylate; BAY 54-9085 |
| Product Type | Chemical |
| Properties | |
| Formula | C21H16ClF3N4O3 . C7H8SO3 |
| MW | 637.03 |
| CAS | 475207-59-1 |
| RTECS | US4588880 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to dark brown powder. |
| Solubility | Soluble in DMSO (5mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | IVDHYUQIDRJSTI-UHFFFAOYSA-N |
| Smiles | ClC1=C(C(F)(F)F)C=C(NC(NC2=CC=C(OC3=CC(C(NC)=O)=NC=C3)C=C2)=O)C=C1.CC4=CC=C(S(=O)(O)=O)C=C4 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
Sorafenib tysolate is a multi-kinase inhibitor that inhibits Raf-1 and B-RAF (IC50s = 6n and 22nM, respectively), as well as the receptor tyrosine kinases VEGFR2, VEGFR3, PDGFRβ, Flt-3, c-Kit, FGFR-1 (Flt-2) and RET, involved in neovascularization and tumor progression. It is selective for these kinases over 12 other kinases, including ERK1, MEK1, EGFR, and HER2 (IC50s = >10μM for all). Sorafenib tosylate exerts broad-spectrum anticancer activity and disrupts signaling pathways that are vital for tumor growth and angiogenesis.
Product References
(1) J.F. Lyons, et al.; Endocr. Relat. Cancer 8, 219 (2001) | (2) S.M. Wilhelm, et al.; Cancer Res. 64, 7099 (2004) | (3) L. Liu, et al.; Cancer Res. 66, 11851 (2006) | (4) D.A. Murphy, et al.; Am. J. Pathol. 169, 1875 (2006) | (5) S. Wilhelm, et al.; Nat. Rev. Drug Discov. 5, 835 (2006) | (6) S.M. Wilhelm, et al.; Mol. Cancer Ther. 7, 3129 (2008) | (7) M.J. Gnoth, et al.; Drug Metab. Dispos. 38, 1341 (2010) | (8) M. Merz, et al.; Eur. J. Cancer. 47, 277 (2011) | (9) S.J. Dixon, et al.; Elife 3, e02523 (2014) | (10) J. Hasskarl; Recent Results Cancer Res. 201, 145 (2014) | (11) L. Wang, et al.; Polymers 15, 2638 (2023) | (12) M. Hendrixson, et al.; Med. Sci. 12, 20 (2024)





