Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
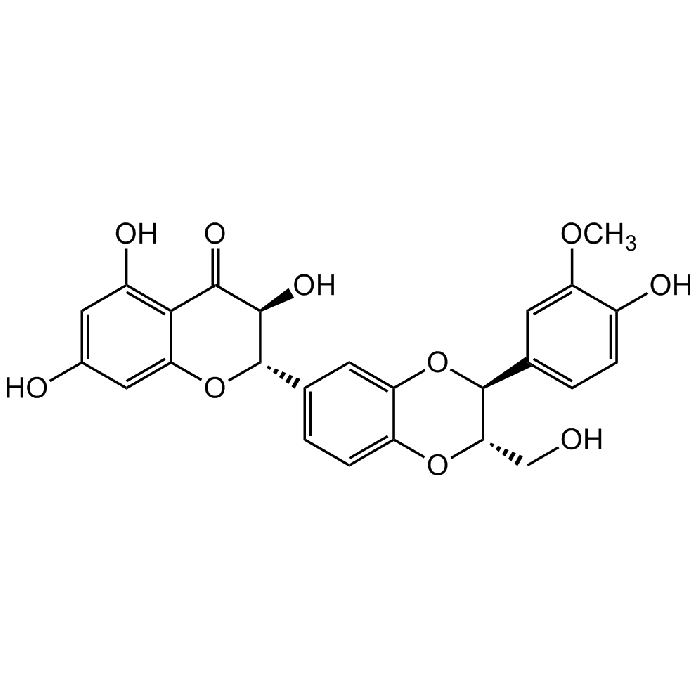
Silibinin
| Product Details | |
|---|---|
| Synonyms | 2,3-Dihydro-3-(4-hydroxy-3-methoxyphenyl)-2-(hydroxymethyl)-6-(3,5,7-trihydroxy-4-oxobenzopyran-2-yl)benzodioxin; Silybin; NSC 651520; Flavobin; Silymarin I |
| Product Type | Chemical |
| Properties | |
| Formula |
C25H22O10 |
| MW | 482.44 |
| CAS | 22888-70-6 |
| RTECS | DJ2981770 |
| Source/Host Chemicals | Plant |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to slightly yellow powder. |
| Solubility | Soluble in DMSO (10mg/ml) or DMF (20mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | SEBFKMXJBCUCAI-PQVVKJAFSA-N |
| Smiles | OC1=C2C(O[C@@H](C3=CC=C(O[C@@H](CO)[C@H](C4=CC=C(O)C(OC)=C4)O5)C5=C3)[C@H](O)C2=O)=CC(O)=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | -20°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Silibinin is a naturally occurring flavanone from Silybum marianum (milk thistle plant) and the main component (active principle) of the Silymarin extract. Silibinin has shown pleiotropic biological properties, including potent antioxidant, anti-inflammatory, anticancer, antidiabetic, hepatoprotective, neuroprotective, antiviral, antimicrobial and immunosuppressive activities. Silibinin demonstrates promising anticancer effects in vitro and in vivo. The molecular mechanisms of silibinin-mediated antiproliferative effects are mainly via receptor tyrosine kinases, androgen receptor, STATs, NF-κB, cell cycle arrest and apoptotis/autophagy inducing signaling pathways in various cancer cells. Silibinin was reported to improve glycemic homeostasis by improving the activity of pancreatic β-cells, increasing insulin sensitivity of liver and muscle cells and decreasing lipid deposition in adipocytes. Next to modulating the NFκ signaling pathway, Sibilin also inhibits NLRP1/NLRP3 inflammasomes signaling pathways. It modulates many molecular changes caused by xenobiotics and ultraviolet radiation to protect the skin and is used in cosmeceutical preparations.
(1) C. Dehmlow, et al.; Life Sci. 58, 1591 (1996) | (2) C. Dehmlow, et al.; Hepatology 23, 749 (1996) | (3) J.A. Hannay & D. Yu; Cancer Biol. Ther. 2, 532 (2003) | (4) R.P. Singh & R. Agarwal; Mutat. Res. 555, 21 (2004) (Review) | (5) R.P. Singh, et al.; Oncogene 24, 1188 (2005) | (6) K. Min, et al.; Arch. Pharm. Res. 30, 1265 (2007) | (7) R.P. Singh & R. Agarwal; Clin. Dermatol. 27, 479 (2009) (Review) | (8) L. Li, et al.; Expert Opin. Investig. Drugs 19, 243 (2010) (Review) | (9) F. Yin, et al.; Neurochem. Int. 58, 399 (2011) | (10) G. Marrazzo, et al.; Neurosci. Lett. 504, 252 (2011) | (11) C.O. Souza, et al.; Life Sci. 91, 159 (2012) | (12) J. McClure, et al.; PLoS One 7, e41832 (2012) | (13) D.R. de Oliveira, et al.; Biomed. Res. Int. 2015, 292797 (2015) | (14) N. Polachi, et al.; Eur. J. Med. Chem. 123, 577 (2016) (Review) | (15) J. Bosch-Barrera, et al.; Cancer Treat Rev. 58, 61 (2017) (Review) | (16) L. Tian, et al.; Microb. Pathog. 108, 104 (2017) | (17) R. Faridnia, et al.; Ann. Parasitol. 64, 29 (2018) | (18) C. Chu, et al.; Arch. Pharm. Res. 41, 785 (2018) (Review) | (19) Z. Jahanafrooz, et al.; Life Sci. 213, 236 (2018) (Review) | (20) M.L. Matias, et al.; Molecules 24, E1548 (2019)





