Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
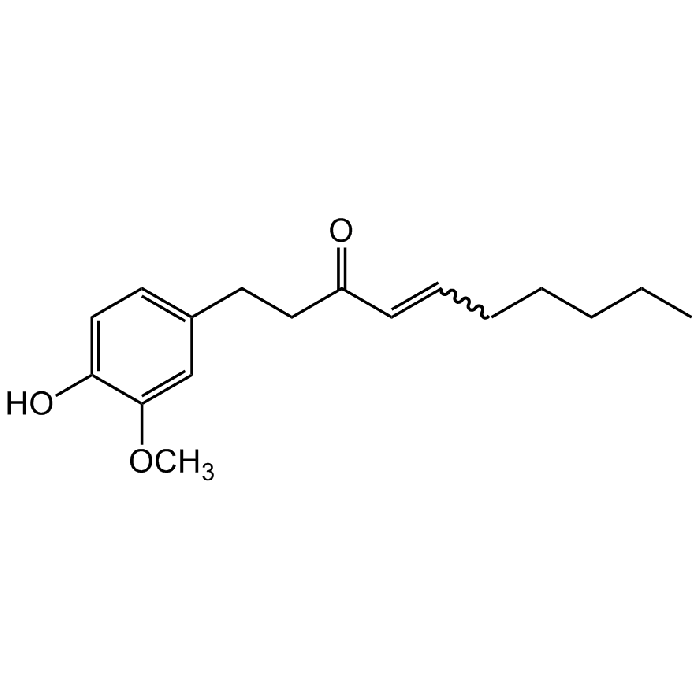
Shogaol
| Product Details | |
|---|---|
| Synonyms | (6)-Shogaol; 1-(4-Hydroxy-3-methoxyphenyl)-4-decen-3-one; CCRIS2038 |
| Product Type | Chemical |
| Properties | |
| Formula |
C17H24O3 |
| MW | 276.37 |
| CAS | 555-66-8 |
| Source/Host Chemicals | Plant |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Colourless to yellow liquid. |
| Solubility | Soluble in DMSO (10mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | OQWKEEOHDMUXEO-BQYQJAHWSA-N |
| Smiles | OC1=CC=C(CCC(/C=C/CCCCC)=O)C=C1OC |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Shogaol is a pungent bioactive component of ginger (Zingiber officinale Roscoe) with anti-inflammatory, anticancer, antioxidant, neuroprotective, antidiabetic, cardioprotective and antimicrobial properties. The anticancer activity is associated with the induction of apoptosis in cancer cells through the production of reactive oxygen species and the activation of caspase, impairment of tubulin polymerization, downregulation of STAT3 and NF-κB signaling, inhibition of the expression of inflammatory mediators and suppresseion of Toll-like receptor signaling. It also has been shown to induce autophagy and activate peroxisomal proliferator activated receptor γ (PPARγ). 6-Shogaol can also activate both TRPV1 and TRPA1 channels. 6-Shogaol inhibits the NF-κB and NLRP3 signaling pathways.
(1) M. Suekawa, et al.; J. Pharmacobiodyn. 7, 836 (1984) | (2) M.H. Pan, et al.; Mol. Nutr. Food Res. 52, 527 (2008) | (3) K. Ishiguro, et al.; FEBS Lett. 582, 3531 (2008) | (4) S.J. Park, et al.; Biosci. Biotechnol. Biochem. 73, 1474 (2009) | (5) J.Y. Hung, et al.; J. Agric. Food Chem. 57, 9809 (2009) | (6) E.P. Sabina, et al.; Food Chem. Toxicol. 48, 229 (2010) | (7) S.K. Ha, et al.; Neuropharmacology 63, 211 (2012) | (8) R. Hu, et al.; PLoS One 7, e39664 (2012) | (9) F. Li, et al.; Food Chem. 135, 332 (2012) | (10) B.S. Tan, et al.; Cancer Lett. 336, 127 (2013) | (11) P. Rebellato & M.S. Islam; JOP 15, 33 (2014) | (12) A. Saha, et al.; Cancer Prev. Res. 7, 627 (2014) | (13) G. Park, et al.; Toxicol. Appl. Pharmacol. 310, 51 (2016) | (14) Y. Xu, et al.; Biomed. Pharmacother. 97, 633 (2018) | (15) J.H. Lee, et al.; Front. Cell Infect. Microbiol. 8, 299 (2018) | (16) T.C. Chen, et al.; Cell Biosci. 10, 5 (2020)





