Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
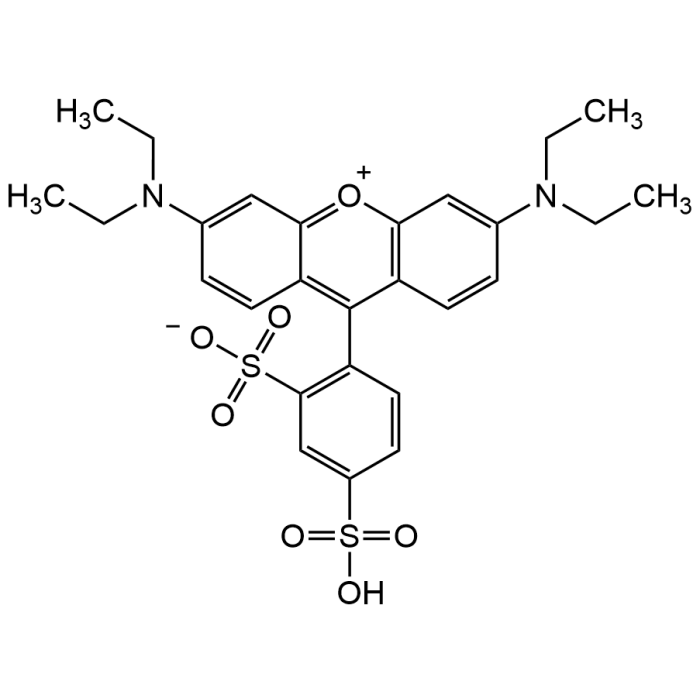
Sulforhodamine B
As low as
39
CHF
CHF 39.00
In stock
Only %1 left
CDX-S0288-G0011 gCHF 39.00
CDX-S0288-G0055 gCHF 142.00
| Product Details | |
|---|---|
| Synonyms | SRB; Lissamine Rhodamine B; SRA-B; Neolan Red E-XB 400 FA |
| Product Type | Chemical |
| Properties | |
| Formula | C27H30N2O7S2 |
| MW | 558.67 |
| CAS | 2609-88-3 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥95% (HPLC) |
| Appearance | Green to brown powder. |
| Solubility | Soluble in DMSO, methanol, ethanol or DMF. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | IOOMXAQUNPWDLL-UHFFFAOYSA-N |
| Smiles | CCN(CC)C1=CC2=[O+]C3=C(C=CC(N(CC)CC)=C3)C(C4=C(S(=O)([O-])=O)C=C(S(=O)(O)=O)C=C4)=C2C=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at RT. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
Sulforhodamine B (free acid) is a xanthene dye widely used in cell biology, analytical chemistry, and histology as a sensitive fluorescent and colorimetric tracer. With strong absorption in the visible range (~565 nm) and intense red emission (~586 nm), it is employed in fluorescence microscopy, flow cytometry, and microplate assays, as well as in cell proliferation and cytotoxicity studies (notably the SRB assay for total cellular protein content). In addition, it is used as a pH indicator, tracer in environmental and hydrological studies. Its stability, high molar absorptivity, and photophysical properties make it a robust, inexpensive, and widely adopted labeling and detection reagent. Spectral Data: λEx=560nm, λEm=580nm.
Product References
(1) P. Skehan, et al.; J. Natl. Cancer Inst. 82, 1107 (1990) | (2) S Marchesini, et al.; Biochem. Int. 27, 545 (1992) | (3) S.P. Fricker; Toxicol. In Vitro 8, 821 (1994) | (4) W. Voigt; Methods Mol. Med. 110, 39 (2005) | (5) V. Vichai & K. Kirtikara; Nat. Protoc. 1, 1112 (2006) | (6) E.A. Orellana & A.L. Kasinski; Bio. Protoc. 6, e1984 (2016)





