Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
Sunitinib malate
As low as
271
CHF
CHF 271.00
In stock
Only %1 left
CDX-S0330-G0011 gCHF 271.00
| Product Details | |
|---|---|
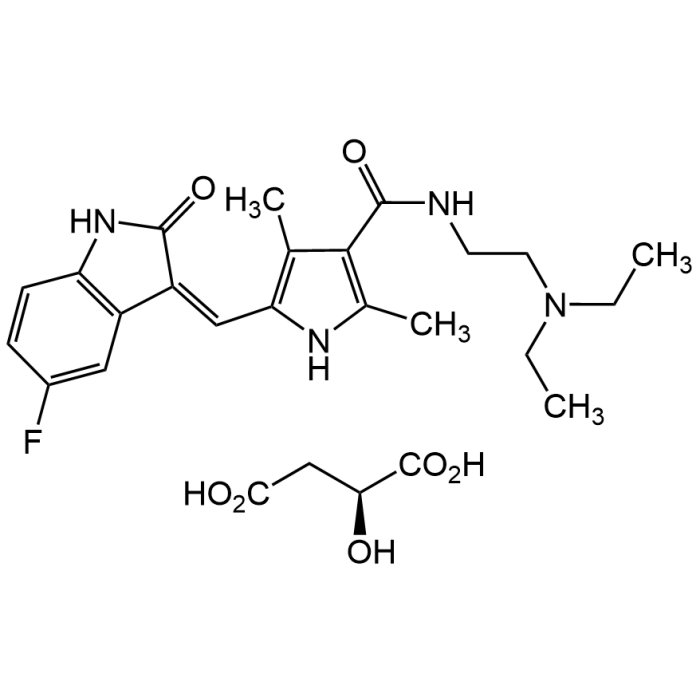
| Synonyms | Sunitinib L-malate; SU112248; N-[2-(Diethylamino)ethyl]-5-[(Z)-(5-fluoro-1,2-dihydro-2-oxo-3H-indol-3-ylidene)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide (2S)-2-hydroxybutanedioic acid (1:1) salt |
| Product Type | Chemical |
| Properties | |
| Formula | C22H27FN4O2 . C4H6O5 |
| MW | 532.56 |
| CAS | 341031-54-7 |
| RTECS | UX9356900 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Yellow, orange to brown powder. |
| Solubility | Soluble in DMSO (10mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | LBWFXVZLPYTWQI-IPOVEDGCSA-N |
| Smiles | CC1=C(/C=C2C(NC3=C\2C=C(F)C=C3)=O)NC(C)=C1C(NCCN(CC)CC)=O.O[C@H](C(O)=O)CC(O)=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at RT. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
Sunitinib malate is a potent ATP-competitive and cell permeable multi-targeted receptor tyrosine kinase (RTK) inhibitor targeting VEGFR, PDGFR-β and c-Kit (Ki = 2-17nM). It inhibits FLK1 (Ki=9nM), PDGFRβ (Ki=8nM) and FLT3. It is at least 10-fold selective for FLK1 and PDGFRβ over a variety of tyrosine kinases in a panel, including EGFR, Cdk2, Met, IGFR-1, Abl and Src. Sunitinib malate inhibits the cellular receptor phosphorylation of FLT3, RET and CSF-1R. Sunitinib malate exhibits potent antiangiogenic and antitumor activity in multiple xenograft models. It decreases VEGF- and FGF-induced proliferation of human umbilical vein endothelial cells (HUVECs) and reduces tumor growth in a variety of mouse xenograft models when administered at doses ranging from 20 to 80 mg/kg per day.
Product References
(1) L. Sun, et al.; J. Med. Chem. 46, 1116 (2003) | (2) D. B. Mendel, et al.; Clin. Cancer Res. 9, 327 (2003) | (3) A.M. O'Farrell, et al.; Blood 101, 3597 (2003) | (4) J. Guo, et al.; Mol. Cancer. Ther. 5, 1007 (2006) | (5) E.D. Deeks & G.M. Keating; Drugs 66, 2255 (2006) | (6) S. Faivre, et al.; Nat. Rev. Drug Discov. 6, 734 (2007) | (7) R. Roskoski; BBRC 356, 323 (2007)





