Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
Selectofluor
As low as
103
CHF
CHF 103.00
In stock
Only %1 left
CDX-S0356-G02525 gCHF 103.00
CDX-S0356-G100100 gCHF 232.00
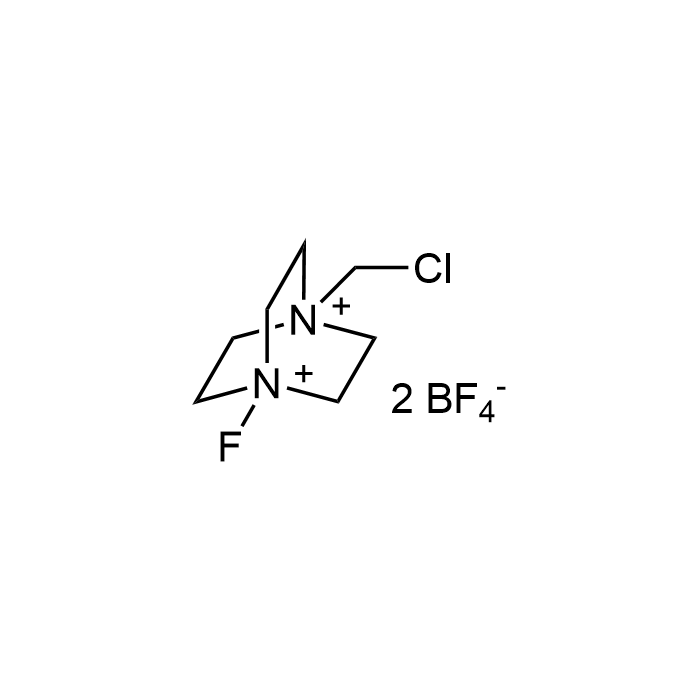
| Product Details | |
|---|---|
| Synonyms | 1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate); F-TEDA-BF4 |
| Product Type | Chemical |
| Properties | |
| Formula | C7H14B2ClF9N2 |
| MW | 354.26 |
| CAS | 140681-55-6 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥98% |
| Appearance | White to off-white powder. |
| Solubility | Soluble in water, DMSO, ethanol or DMF. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | REEJSGMVBVSCDW-UHFFFAOYSA-N |
| Smiles | ClC[N+]12CC[N+](CC2)(F)CC1.F[B-](F)(F)F.F[B-](F)(F)F |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
Selectfluor (F-TEDA-BF4) is a powerful, stable, and easy-to-handle electrophilic fluorinating reagent widely used in organic synthesis. It enables the direct introduction of fluorine into a broad range of substrates, including aromatics, heteroaromatics, alkenes, and activated C-H bonds, under mild conditions with high selectivity. Selectfluor also functions as a strong selective oxidizing agent and has applications in oxidative coupling, halogenation, and late-stage functionalization of complex molecules.
Product References
(1) R.E. Banks; J. Chem. Soc. Chem. Commun. 1994, 343 (1994) | (2) G. Sankar Lal, et al.; J. Org. Chem. 60, 7340 (1995) | (3) R.E. Banks; J. Chem. Soc. Perkin Trans. 1, 2069 (1996) | (4) G. Sankar Lal, et al.; Chem. Rev. 96, 1737 (1996) | (5) R.E. Banks; J. Fluorine Chem. 87, 1 (1998) | (6) G. Stavber, et al.; Org. Lett. 6, 4973 (2004) | (7) P.T. Nyffeler, et al.; Angew. Chem. Int Ed. 44, 192 (2005) | (8) L. Manral; Synlett Spotlight 155, 807 (2006) | (9) G. Stavber; Molecules 26, 6432 (2011) | (10) T.C Allmann, et al.; Chemistry 22, 111 (2016) | (11) J.D. Galloway, et al.; Org. Lett. 19, 5772 (2017)





