Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
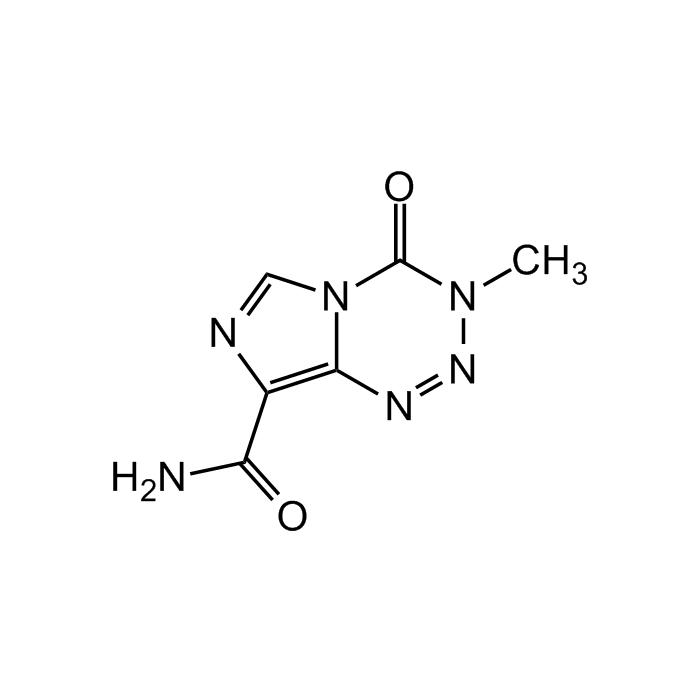
Temozolomide
| Product Details | |
|---|---|
| Synonyms | 3,4-Dihydro-3-methyl-4-oxoimidazo[5,1-d]-1,2,3,5-tetrazine-8-carboxamide; CCRG 81045; MB 39831; Methazolastone; NSC 362856; Temodal |
| Product Type | Chemical |
| Properties | |
| Formula |
C6H6N6O2 |
| MW | 194.15 |
| CAS | 85622-93-1 |
| RTECS | NJ5927050 |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to light pink powder. |
| Solubility | Soluble in DMSO (5mg/ml) or DMF (5mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | BPEGJWRSRHCHSN-UHFFFAOYSA-N |
| Smiles | O=C1N(C)N=NC2=C(C(N)=O)N=CN21 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Temozolomide is a DNA methylating agent and drug resistance-modifying agent with antitumor and antiangiogenic properties. Temozolomide induces G2/M arrest and apoptosis through adduction of a methyl group to O6 position of guanine in genomic DNA and interfering with DNA replication and leading to cytotoxicity in proliferating cells. It has been shown to induce autophagy in malignant glioma cells. It is effective in a variety of types of cancer, including aggressive brain cancers.
(1) A.S. Clark, et al.; J. Med. Chem. 38, 1493 (1995) | (2) E.S. Newlands, et al.; Cancer Treat. Rev. 23, 35 (1997) (Review) | (3) S.S. Agarwala & J.M. Kirkwood; Oncologist 5, 144 (2000) (Review) | (4) S.J. Danson & M.R. Middleton; Expert Rev. Anticancer Ther. 1, 13 (2001) (Review) | (5) T. Kanzawa, et al.; Cell Death Differ. 11, 448 (2004) | (6) F. Marchesi, et al.; Pharmacol. Res. 56, 275 (2007) | (7) G. Jiang, et al.; Curr. Med. Chem. 19, 3886 (2012) (Review)





