Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
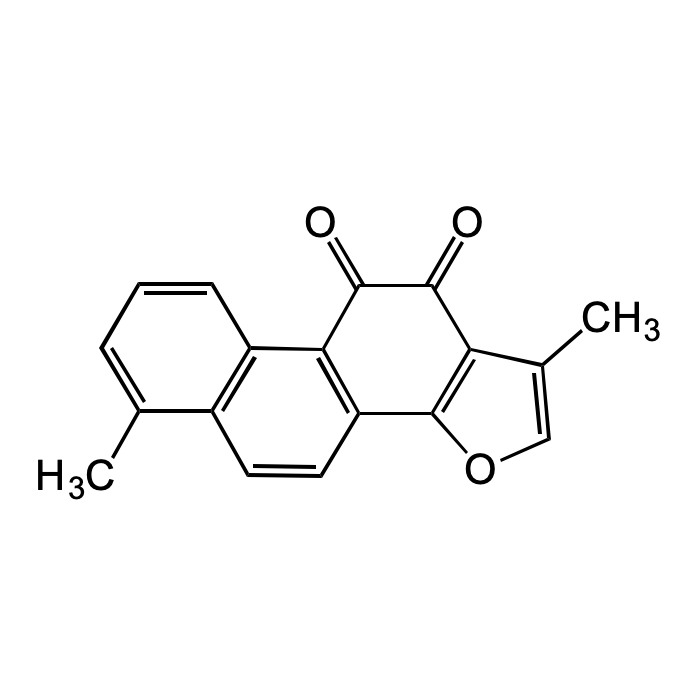
Tanshinone I
| Product Details | |
|---|---|
| Synonyms | Tanshinon I; Tanshinone A; 1,6-Dimethylphenanthro[1,2-b]furan-10,11-dione |
| Product Type | Chemical |
| Properties | |
| Formula | C18H12O3 |
| MW | 276.29 |
| CAS | 568-73-0 |
| Source/Host Chemicals | Isolated from plant source. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Purple-black powder. |
| Solubility | Soluble in DMSO, chloroform or methanol. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | AIGAZQPHXLWMOJ-UHFFFAOYSA-N |
| Smiles | O=C(C1=C(C2=C3C(C)=CO2)C=CC4=C1C=CC=C4C)C3=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Tanshinone I is a diterpene that has been found in S. miltiorrhiza and has diverse biological activities, including anticancer, antidiabetic, anti-inflammatory, immunosuppressive, anticoagulant and neuroprotective properties. It inhibits proliferation in a variety of cancer cell lines, including KB/VCR, MCF-7/ADR, and K562/A02 multidrug resistance cell lines. It inhibits topoisomerase I- and topoisomerase II-mediated supercoiled DNA relaxation activity. It has been shown to induce apoptosis in several cancer cell lines. It reduces plasma levels of total cholesterol, triglycerides, LDL and nonesterified fatty acids and decreases blood glucose levels in a rat model of type 2 diabetes induced by streptozotocin and a high-fat diet. Tanshinone I reduces the LPS-induced expression of TNF-α, IL-1β, and IL-6 in microglia in vitro. It is neuroprotective in a mouse model of Parkinson‘s disease induced by MPTP.
(1) J.Y. Kim, et al.; Pharmacol. Toxicol. 92, 195 (2003) | (2) I.T. Nizamutdinova, et al.; Carcinogenesis 29, 1885 (2008) | (3) I.T. Nizamutdinova, et al.; Int. J. Oncol. 33, 485 (2008) | (4) C.Y. Lee, et al.; Mol. Cancer Ther. 7, 3527 (2008) | (5) L. Yang, et al.; PLoS One 8, e80464 (2013) | (6) J.H. Park, et al.; Neurochem. Res. 39, 1300 (2014) | (7) S. Wang, et al.; J. Ethnaopharmacol. 164, 247 (2015) | (8) M.K. Kim, et al.; Fitoterapia 101, 162 (2015) | (9) X. Jing, et al.; Neurochem. Res. 41, 779 (2016) | (10) Y. Wei, et al.; Biomed. Pharmacother. 93, 352 (2017) | (11) Q.-T. Tian, et al.; Biochem. Pharmacol. 156, 255 (2018) | (12) Y. Yan, et al.; Mol. Pharm. 15, 4843 (2018)





