Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
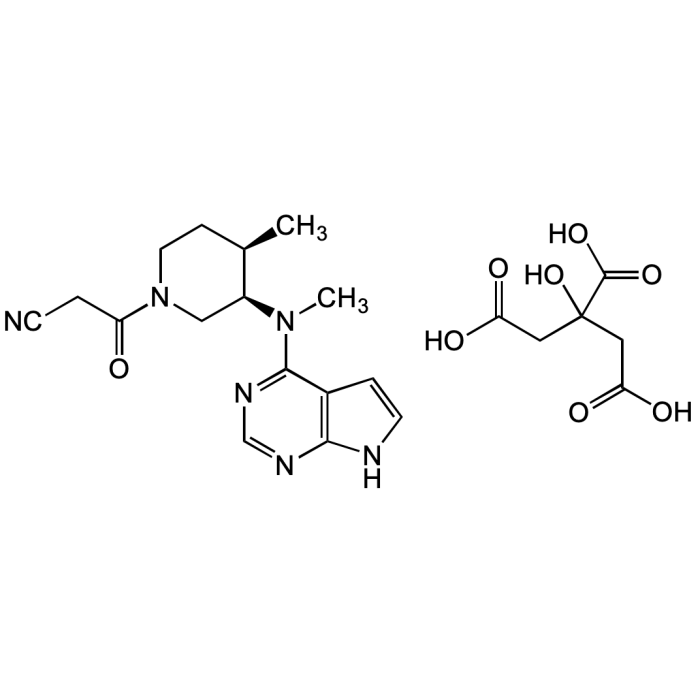
Tofacitinib citrate
| Product Details | |
|---|---|
| Synonyms | CP-690550; Xeljanz; Tasocitinib; (3R,4R)-4-Methyl-3-(methyl-7H-pyrrolo[2,3-d]pyrimidin-4-ylamino)-β-oxo-1-piperidinepropanenitrile citrate salt |
| Product Type | Chemical |
| Properties | |
| Formula | C16H20N6O . C6H8O7 |
| MW | 312.4 . 192.1 |
| CAS | 540737-29-9 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in DMSO (20mg/ml) or DMF (5 mg/ml). Slightly soluble in ethanol. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | SYIKUFDOYJFGBQ-YLAFAASESA-N |
| Smiles | OC(CC(O)=O)(CC(O)=O)C(O)=O.O=C(CC#N)N1C[C@@H]([C@H](C)CC1)N(C)C2=NC=NC3=C2C=CN3 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at RT. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Tofacitinib citrate is a potent cell permeable JAK3 inhibitor (IC50=1nM), which Shows also JAK-1 inhibitory activity. This is an immunosuppressive and anti-inflammatory agent. It inhibits signaling through heterodimeric receptors associated with JAK3, JAK1, or both of them, with functional selectivity over JAK2-paired receptors. Blocks downstream STAT signaling, resulting in potent inhibition of several cytokines, including interleukins 2, 4, 7, 9, 15 and 21, which are integral to lymphocyte activation, function and proliferation. It also has been shown to be a potent and selective inhibitor of HIV-1 replication and virus reactivation in vitro. It is investigated against the spread of the SARS-CoV-2 (COVID-19).
(1) P.S. Changelian, et al.; Science 302, 875 (2003) | (2) D.C. Borie, et al.; Transplantation 79, 791 (2005) | (3) D.C. Borie, et al.; Transplantation 80, 1756 (2005) | (4) K. West; Curr. Opin. Investig. Drugs 10, 491 (2009) (Review) | (5) M.E. Flanagan, et al.; J. Med. Chem. 53, 8468 (2010) | (6) W. Ju, et al.; Blood 117, 1938 (2011) | (7) C. Gavegnano, et al.; Antimicrob. Agents Chemother. 58, 1977 (2014) | (8) S.P. Wang, et al.; Ann. Rheum. Dis. 73, 2213 (2014)





