Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
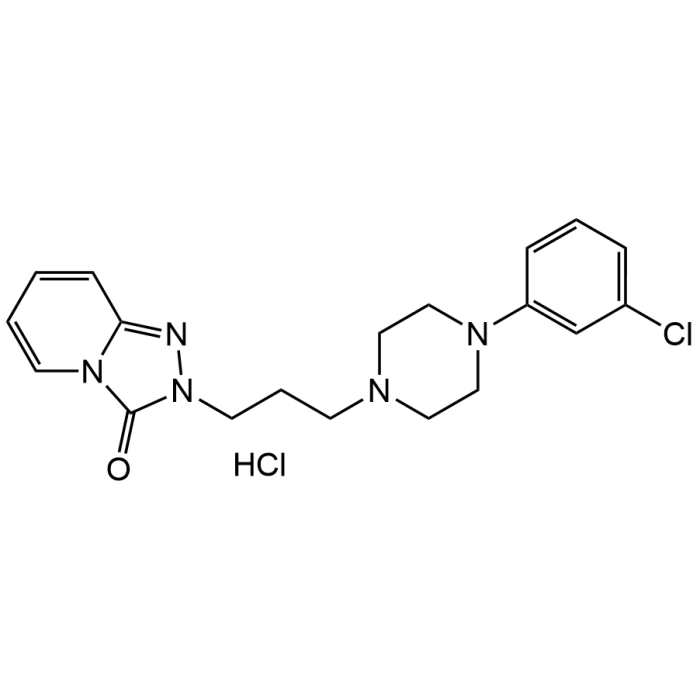
Trazodone hydrochloride
As low as
45
CHF
CHF 45.00
In stock
Only %1 left
CDX-T0476-G0011 gCHF 45.00
CDX-T0476-G0055 gCHF 103.00
| Product Details | |
|---|---|
| Synonyms | 2-[3-[4-(3-Chlorophenyl)-1-piperazinyl]propyl]-1,2,4-triazolo[4,3-a]pyridin-3(2H)-one hydrochloride; AF 1161; Desyrel; Molipaxin; KB 831; Pragmazone; Thombran; Trazolan; Tombran; Trittico; Bimaran; Mesyrel |
| Product Type | Chemical |
| Properties | |
| Formula | C19H22ClN5O . HCl |
| MW | 408.32 |
| CAS | 25332-39-2 |
| RTECS | XZ5660000 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥99% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in DMSO (10mg/ml), methanol (10mg/ml) or water (1mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | OHHDIOKRWWOXMT-UHFFFAOYSA-N |
| Smiles | ClC1=CC=CC(N(CC2)CCN2CCCN3N=C(C=CC=C4)N4C3=O)=C1.Cl |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
Trazodone hydrochloride belongs to the class of serotonin receptor antagonists and reuptake inhibitors (SARIs). Trazodone hydrochloride has anti-depressant and neuroprotectant activity. Trazodone hydrochloride exerts antagonistic properties against α1- and α2-adrenergic receptors and histamine H1 receptors, with minimal anticholinergic effects. Shown to enhance neuronal differentiation of mouse and human neural progenitor cells. Also inhibits PERK/eIF2α-P-mediated reduction in protein synthesis and restores memory deficits in a mouse dementia model. Its broad pharmacological activity makes it a valuable tool in both neuroscience research and therapeutic development. Can be used as a reference compound.
Product References
(1) A. Georgotas, et al.; Pharmacotherapy 2, 255 (1982) | (2) S.G. Bryant & L. Ereshefsky; Clin. Pharm. 1, 406 (1982) | (3) A. Fagiolini, et al.; Review CNS Drugs 26, 1033 (2012) | (4) M. Halliday, et al.; Brain 140, 1768 (2017) | (5) V. Bortolotto, et al.; ACS Chem. Neurosci. 8, 2027 (2017)





