Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
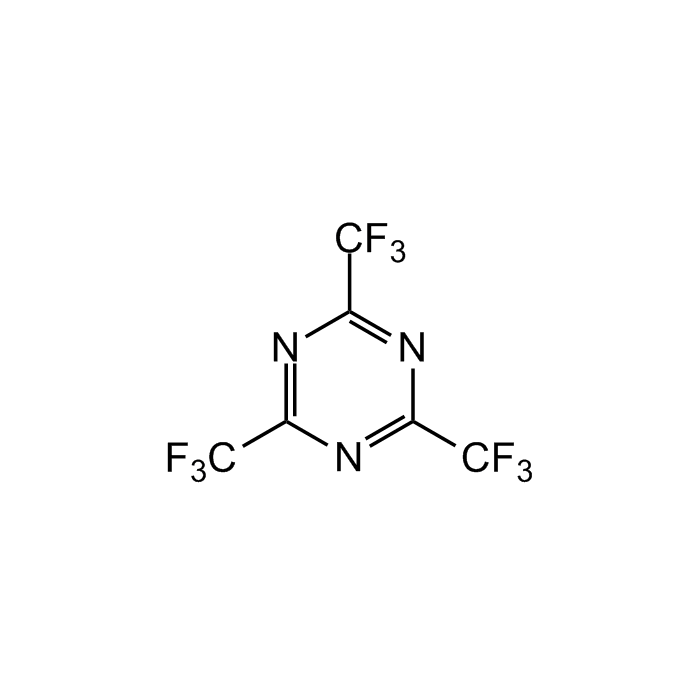
2,4,6-Tris(trifluoromethyl)-1,3,5-triazine
| Product Details | |
|---|---|
| Synonyms | TTFMT |
| Product Type | Chemical |
| Properties | |
| Formula |
C6F9N3 |
| MW | 285.07 |
| CAS | 368-66-1 |
| RTECS | XZ2800000 |
| Purity Chemicals | ≥98.5% (GC) |
| Appearance | Colourless liquid. |
| Solubility | Soluble in chloroform, DCM or DMSO. |
| Identity | Determined by 19F-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | LSGBKABSSSIRJF-UHFFFAOYSA-N |
| Smiles | FC(F)(F)C1=NC(C(F)(F)F)=NC(C(F)(F)F)=N1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Handling Advice |
Keep under inert gas. Very hygroscopic. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Heterocyclic fluoride-rich triazine building block and intermediate for synthesis of dyes and probes. Also used as an analytical reference compound for chromatography. 2,4,6-Tris(trifluoromethyl)-1,3,5-triazine is used as a secondary battery electrolyte in power storage systems and as a flame retardant additive that can improve both the safety and the performance of Li-ion batteries. It shows excellent thermal stability with charged cathodes and anodes. A high degree of fluorination may help to deliver better performance in terms of the cycle life and the rate capability, in the same way as has been shown for other fluorinated additives. Triazines are well known as flame retardant materials, as crosslinkers for polymer synthesis, for battery applications they have been used as a water/acid scavenger.
(1) T.R. Norton; JACS 72, 8 (1950) | (2) K. Kim, et al.; Electroch. Acta 54, 2259 (2009) | (3) V.O. Iaroshenko, et al.; Chemistry 17, 7188 (2011) | (4) M.D. Rosa, et al.; J. Org. Chem. 78, 8614 (2013) | (5) Z. Huang, et al.; RSC Adv. 4, 13434 (2014) | (6) T. Hiasa, et al.: Jpn. Kokai Tokkyo Koho, 77 (2018)





