Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
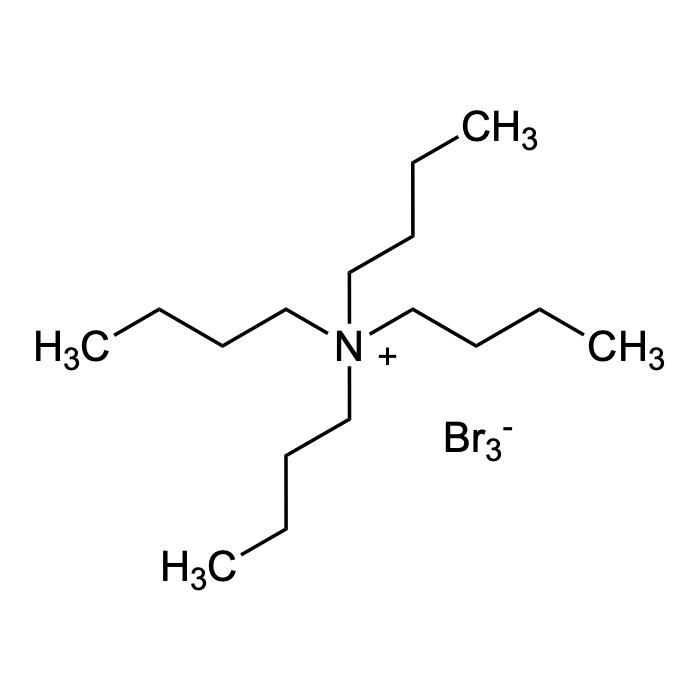
Tetrabutylammonium tribromide
| Product Details | |
|---|---|
| Synonyms | TBABr3; Tetrabutylammoniumbromide-perbromide; N,N,N,N-Tetrabutylammonium tribromide |
| Product Type | Chemical |
| Properties | |
| Formula | C16H36Br3N |
| MW | 482.18 |
| CAS | 38932-80-8 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥98% (T) |
| Appearance | Dark yellow to dark orange powder. |
| Solubility | Soluble in acetonitrille, chloroform, THF, dioxane. Insoluble in water. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | XXSLZJZUSYNITM-UHFFFAOYSA-N |
| Smiles | Br[Br-]Br.CCCC[N+](CCCC)(CCCC)CCCC |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at RT. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Tetrabutylammonium tribromide (TBABr3) is used as a reagent in organic synthesis, particularly in organic and organometallic chemistry. It is a quaternary ammonium salt and is composed of a tetrabutylammonium cation (C16H36N+) and three bromide anions (Br-). TBABr3 is a powerful brominating agent, to introduce bromine (Br) atoms into organic molecules, and it is particularly useful for bromination reactions in cases where other brominating agents might not be suitable or effective. The tetrabutylammonium cation in TBABr3 serves as a phase-transfer catalyst, facilitating the transfer of the bromide ions into the organic phase of a reaction. This allows for the selective bromination of various organic substrates. TBABr3 is used in a variety of organic reactions, including the preparation of intermediates for pharmaceuticals, and in other applications where the introduction of bromine is required.
(1) R. Gopinath & B.K. Patel; Org. Lett. 2, 4177 (2000) | (2) S. Naik, et al.; Org. Biomol. Chem. 2, 1670 (2004) | (3) X. Lin, et al.; Synth. Commun. 36, 3153 (2006) | (4) A.T. Khan, et al.; Carbohydr. Res. 346, 673 (2011) | (5) S. Gao, et al.; J. Org. Chem. 83, 9250 (2018) | (6) M. Belal, et al.; Org. Biomol. Chem. 20, 2562 (2022)





