Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
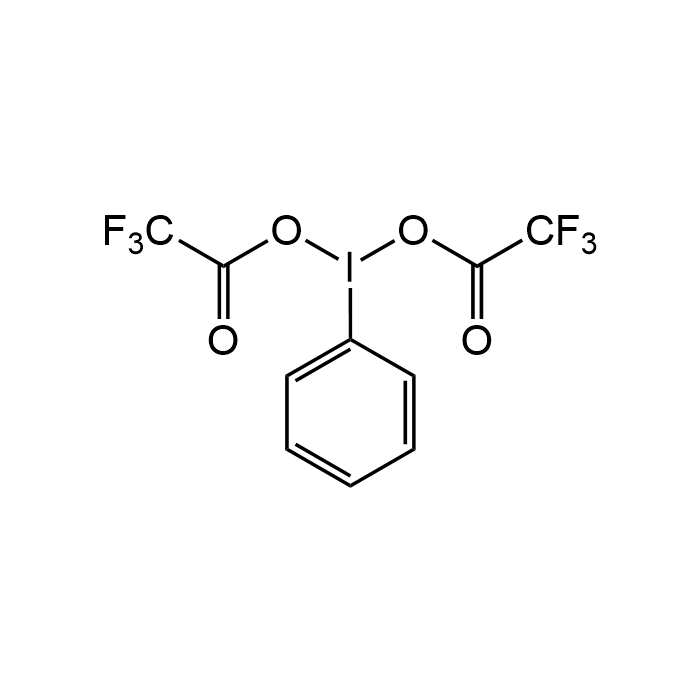
(Bis(trifluoroacetoxy)iodo)benzene
As low as
64
CHF
CHF 64.00
In stock
Only %1 left
CDX-T0620-G01010 gCHF 64.00
CDX-T0620-G05050 gCHF 155.00
| Product Details | |
|---|---|
| Synonyms | BTIB; PIFA; Phenyliodine bis(trifluoroacetate) |
| Product Type | Chemical |
| Properties | |
| Formula | C10H5F6IO4 |
| MW | 430.04 |
| CAS | 2712-78-9 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥95% (AT) |
| Appearance | Colorless to white powder or crystals. |
| Solubility | Soluble in chloroform. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | PEZNEXFPRSOYPL-UHFFFAOYSA-N |
| Smiles | O=C(C(F)(F)F)OI(OC(C(F)(F)F)=O)C1=CC=CC=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
(Bis(trifluoroacetoxy)iodo)benzene (BTIB; PIFA) is a hypervalent iodine reagent widely used as a mild and selective oxidizing agent in organic synthesis. It facilitates oxidative transformations such as phenol coupling, dearomatization, α-oxygenation of carbonyl compounds, and heterocycle formation under mild conditions, often avoiding harsh metal-based oxidants. (Bis(trifluoroacetoxy)iodo)benzene is also employed in peptide and carbohydrate chemistry for oxidative cleavage and functionalization of sensitive substrates.
Product References
(1) P.V. Pallai, et al.; Int. J. Pept. Protein Res. 21, 84 (1983) | (2) P.V. Pallai, et al.; Biochemistry 24, 1933 (1985) | (3) M. Catir, et al.; J. Org. Chem. 74, 4560 (2009) | (4) D. Kumar, et al.; Bioorg. Med. Chem. Lett. 19, 4492 (2009) | (5) N. Chatterjee, et al.; Org. Biomol. Chem. 13, 4828 (2015) | (6) T. Imai, et al.; J. Org. Chem. 81, 3975 (2016) | (7) Y. Wan, et al.; J. Org. Chem. 84, 780 (2019) | (8) C. Mudithanapelli, et al.; Org. Lett. 21, 3098 (2019) | (9) C. Mudithanapelli & M.H. Kim; Org. Biomol. Chem. 18, 450 (2020) | (10) X.T. Sun, et al.; Molecules 27, 726 (2022) | (11) Y. Matsuo, et al.; Angew. Chem. Int. Ed. Engl. 62, e202314968 (2023)





