Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
Umbelliferon
| Product Details | |
|---|---|
| Synonyms | 7-Hydroxycoumarin; 7-Hydroxy-2H-1-benzopyran-2-one; NSC 19790 |
| Product Type | Chemical |
| Properties | |
| Formula |
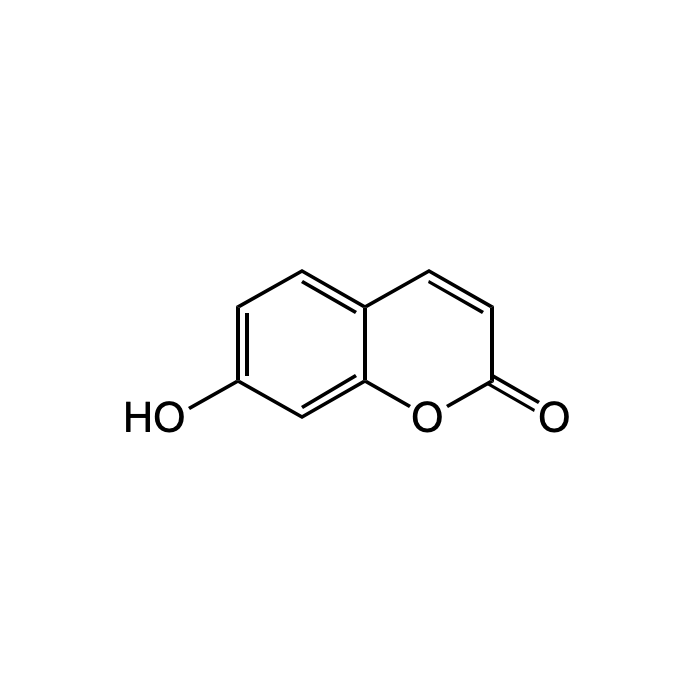
C9H6O3 |
| MW | 162.14 |
| CAS | 93-35-6 |
| RTECS | GN6820000 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥98% (NMR) |
| Appearance | Beige solid. |
| Solubility | Soluble in DMSO (10mg/ml), ethanol (5 mg/ml), methanol (5 mg/ml) or dioxane. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | ORHBXUUXSCNDEV-UHFFFAOYSA-N |
| Smiles | O=C1OC2=CC(O)=CC=C2C=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Umbelliferone is a naturally occurring derivative and metabolite of coumarin that has diverse biological activities including antitumor, antioxidant, antihyperglycemic, anti-inflammatory, neuroprotective and antidepressant-like properties. It inhibits the growth of a variety of human cell lines. It increases the production of reactive oxygen species (ROS), depolarizes the mitochondrial membrane, and halts the cell cycle at the G0/G1 phase in KB human oral carcinoma cells. Umbelliferone has been used as a ratiometric pH indicator. Spectral Data: Em=460nm / Ex=330(ph<6)/370nm(pH>8). Umbelliferone can also be used as building block to synthesize fluorescent probes or biochemically active APIs.
(1) D.W. Fink & W.R. Koehler; Anal. Chem. 42, 990 (1970) | (2) M.E. Marshall, et al.; J. Cancer Res. Clin. Oncol. 120, S3 (1994) | (3) B. Ramesh & K.V. Pugalendi; J. Med. Food 9, 562 (2006) | (4) K.S. Lee, et al.; Org. Lett. 10, 49 (2008) | (5) K. Iliopoulos, et al.; JACS 132, 14343 (2010) | (6) S.R. Subramaniam & E.M. Ellis; J. Neurosci. Res. 91, 453 (2013) | (7) A. Rauf, et al.; Nat. Prod. Res. 28, 1371 (2014) | (8) T. Qin, et al.; Behav. Brain Res. 317, 147 (2017) | (9) A. Vijayalakshmi & G. Sindhu; Biomed. Pharmacother. 92, 661 (2017) | (10) X. Wang, et al.; Acta Pharm. 69, 111 (2019)





