Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
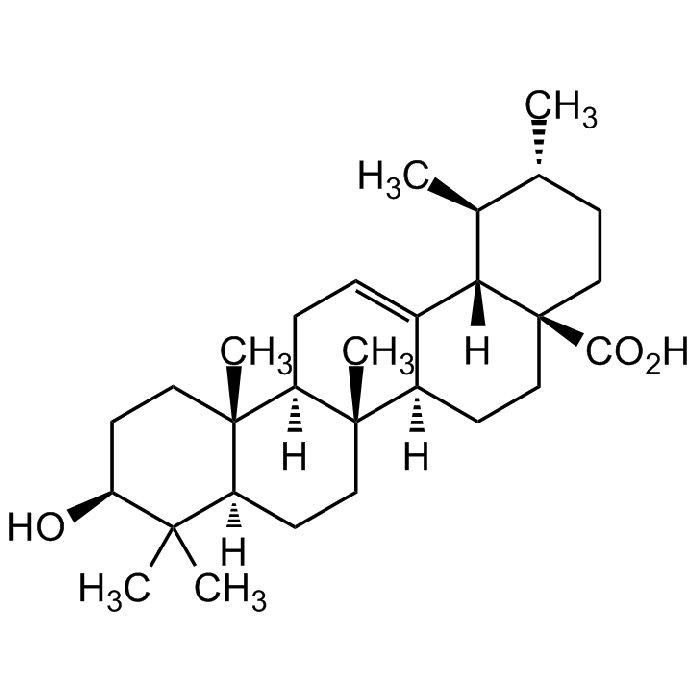
Ursolic acid
| Product Details | |
|---|---|
| Synonyms | 3β-Hydroxy-12-ursen-28-oic acid; Bungeolic Acid; Maerotaine; Malol; NSC 4060; NSC 167406 |
| Product Type | Chemical |
| Properties | |
| Formula |
C30H48O3 |
| MW | 456.711 |
| CAS | 77-52-1 |
| Source/Host Chemicals | Plant |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White Powder. |
| Solubility | Soluble in DMSO (10mg/ml) or DMF (10mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | NZFQEZMPDNBQMO-PBILNHPASA-N |
| Smiles | C[C@@H]1CC[C@]2(C(O)=O)CC[C@@]3([H])[C@]4(C)CC[C@@]5([H])C(C)(C)[C@@H](O)CC[C@]5(C)[C@@]4([H])CC=C3[C@]2([H])[C@H]1C |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Ursolic acid is a pentacyclic triterpenoid that has been isolated from M. pumila. Ursolic acid has diverse biological effects, including anti-inflammatory, anticancer, antidiabetic, antiobesity, neuroprotective, cardioprotective, hepatoprotective, antioxidant and antiviral and antibacterial effects. Effects relevant to its anticancer properties include, apoptosis and autophagy induction, preventing angiogenesis, microtubule-destabilizing, induction of cell cycle arrest and inhibition of tumor growth. Ursolic acid inhibits NF-κ signaling through suppression of IκBα kinase and p65 phosphorylation. It has been shown to enhance pancreatic beta-cell function in diabetic mice and activates the TGR5 receptor to enhance GLP-1 secretion. Its antiobesity potential is related to inhibiting pancreatic lipase and adipogenesis and increasing insulin sensitivity and inhibiting α-glucosidase. Ursolic acid is also liver X receptor α (LXRα) antagonist inhibiting ligand-induced Nonalcoholic fatty liver and drug-induced lipogenesis. Ursolic acid also scavenges 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals and inhibits the growth of several strains of Gram-positive and Gram-negative bacteria in vitro.
(1) Z. Kowalewski, et al.; Arch. Immunol. Ther. Exp. 24, 115 (1976) | (2) D.K. Kim, et al.; Int. J. Cancer 87, 629 (2000) | (3) L. Novotny, et al.; Neoplasma 48, 241 (2001) (Review) | (4) F. Abe, et al.; Biol Pharm Bull. 25, 1485 (2002) | (5) S. Shishodia, et al.; Cancer Res. 63, 4375 (2003) | (6) M.S. Ali, et al.; Phytother. Res. 21, 558 (2007) | (7) A. Nataraju, et al.; Curr. Top Med. Chem. 7, 801 (2007) | (8) S.M. Jang, et al.; Int. Immunopharmacol. 9, 113 (2009) | (9) M. Kanjoormana & G. Kuttan; Integr. Cancer Ther. 9, 224 (2010) | (10) I. Kazmi, et al.; Eur. J. Pharmacol. 709, 28 (2013) | (11) Y. He, et al.; PLoS One 8, e70135 (2013) | (12) L. Wozniak, et al.; Molecules 20, 20614 (2015) (Review) | (13) D. Kashyap, et al.; Recent Pat. Inflamm. Allergy Drug Discov. 10, 21 (2016) (Review) | (14) C.K. Katashima, et al.; Obes. Rev. 18, 700 (2017) (Review) | (15) A.M. Ramirez-Rodriguez, et al.; J. Med. Food. 20, 882 (2017) | (16) S.H. Lo, et al.; Naunyn Schmiedebergs Arch. Pharmacol. 390, 1097 (2017) | (17) Y.N. Lin, et al.; J. Agric. Food Chem. 66, 11647 (2018) | (18) S. Habtemariam; Oxid. Med. Cell Longev. 2019, 8512048 (2019) (Review) | (18) S. Mlala, et al.; Molecules 24, E2751 (2019) (Review) | (20) J. Wang, et al.; J. Sci. Food Agric. 100, 986 (2020)





