Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
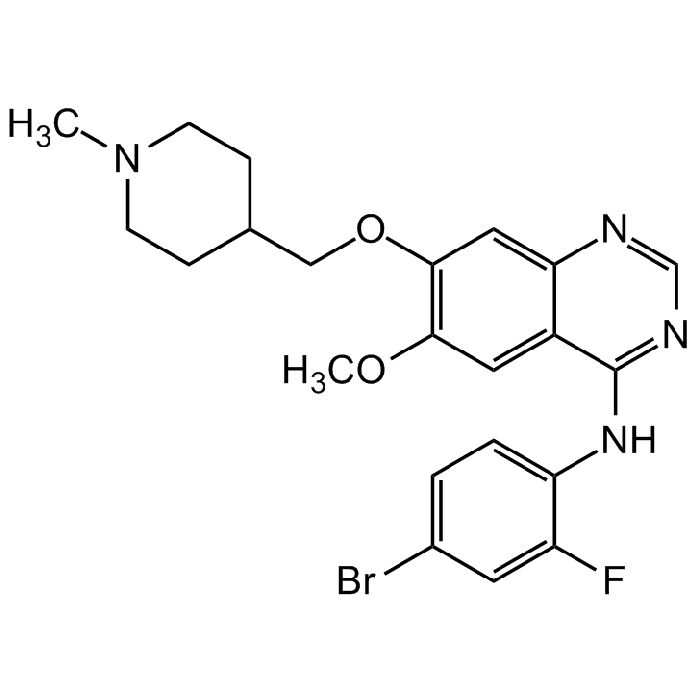
Vandetanib
| Product Details | |
|---|---|
| Synonyms | N-(4-Bromo-2-fluorophenyl)-6-methoxy-7-[(1-methyl-4-piperidinyl)methoxy]-4-quinazolinamine; ZD6474; Zactima; CH 331 |
| Product Type | Chemical |
| Properties | |
| Formula |
C22H24BrFN4O2 |
| MW | 475.4 |
| CAS | 443913-73-3 |
| RTECS | VA0918650 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥98% (NMR) |
| Appearance | White to beige powder. |
| Solubility | Soluble in DMSO or DMF (5mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | UHTHHESEBZOYNR-UHFFFAOYSA-N |
| Smiles | BrC(C=C1F)=CC=C1NC2=NC=NC3=CC(OCC4CCN(C)CC4)=C(OC)C=C32 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Vandetanib is an orally available, ATP mimetic small molecule tyrosine kinases inhibitor that targets vascular endothelial growth factor receptor-2 (VEGFR-2), VEGFR-3 and epidermal growth factor receptor (EGFR), REarranged during Transfection (RET) and slightly VEGFR-1. Inhibition of these tyrosine kinases blocks multiple intracellular signaling pathways involved in tumor growth, proliferation, progression and angiogenesis. It inhibits RET, VEGFR2, VEGFR3, VEGFR1, EGFR, PDGFRβ, Tie-2, and FGFR1 in cell-free assays (IC50s = 34, 40, 110, 1,600, 500, 1,100, 2,500, and 3,600 nM, respectively). It also binds to 142 additional kinases in a panel of 442 kinases (Kds = 4.6-7,900 nM). Vandetanib (1 and 2.5 µM) induces apoptosis, autophagy, ROS and cell cycle arrest at the G0/G1 phase and has anti-proliferative properties in several cancer cell lines and in in vivo cancer models. Formulations containing vandetanib have been used in the treatment of medullary thyroid cancer.
(1) L.F. Hennequin, et al.; J. Med. Chem. 45, 1300 (2002) | (2) F. Carlomagno, et al.; Cancer Res. 62, 7284 (2002) | (3) S.R. Wedge, et al.; Cancer Res. 62, 4645 (2002) | (4) F. Ciardiello, et al.; Clin. Cancer Res. 9, 1546 (2003) | (5) J.N. Rich, et al.; Clin. Cancer Res. 11, 8145 (2005) | (6) S. Sathornsumetee & J.N. Rich; Drugs Today 42, 657 (2006) (Review) | (7) R.S. Herbst, et al.; Expert Opin. Investig. Drugs 16, 239 (2007) (Review) | (8) A. Morabito, et al.; Oncologist 14, 378 (2009) (Review) | (9) M.I. Davis, et al.; Nat. Biotechnol. 29, 1046 (2011) | (10) K. Inoue, et al.; Clin. Cancer Res. 18, 3924 (2012) | (11) G.M. Keating, et al.; BioDrugs 26, 431 (2012) (Review) | (12) S. Jasim, et al.; Biologics 8, 281 (2014) | (13) M.W. Sim & M.S. Cohen; Expert Opin. Drug Discov. 9, 105 (2014) (Review)





