Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
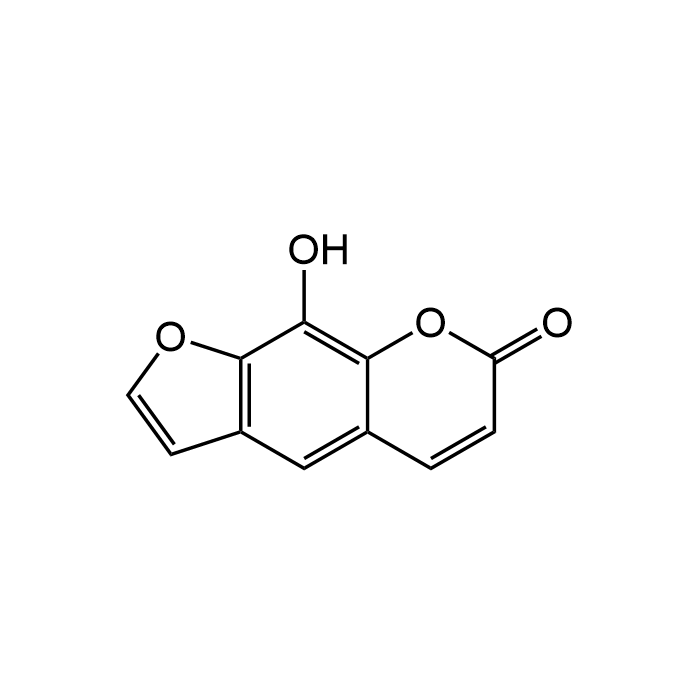
Xanthotoxol
| Product Details | |
|---|---|
| Synonyms | 8-Hydroxypsoralen |
| Product Type | Chemical |
| Properties | |
| Formula | C11H6O4 |
| MW | 202.16 |
| CAS | 2009-24-7 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥97% (NMR) |
| Appearance | White to yellow powder. |
| Solubility | Soluble in DMSO. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | JWVYQQGERKEAHW-UHFFFAOYSA-N |
| Smiles | OC1=C2C(C=CC(O2)=O)=CC3=C1OC=C3 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Xanthotoxol is a coumarin and a major component in C. monnieri that has diverse biological activities, including anti-inflammatory, antioxidant, neuroprotective or anti-proliferative properties. It inhibits proliferation of HeLa and HepG2 cells in vitro. Xanthotoxol increases histamine release from mast cells and decreases secretion of TNF-α, IL-4, and IL-1β from RBL-2H3 cells induced by DNP-human serum albumin (DNP-HSA). It is nematocidal against M. incognita. Xanthotoxol reduces brain edema, neutrophil infiltration, blood-brain barrier disruption, production of IL-1β, TNF-α, IL-8, and nitric oxide (NO), and levels of intercellular adhesion molecule-1 (ICAM-1) and E-selectin in a rat model of focal cerebral ischemia. Xanthotoxol promoted NSCLC cell cycle arrest and apoptosis, and inhibited EMT by suppressing the activity of PI3K-AKT pathway, thus attenuating NSCLC proliferation and metastasis. Xanthotoxol provides itch relieve in mice by suppressing spinal GRP/GRPR signaling. This compound can be used as internal analytical reference material.
(1) O.P. Sethi, et al.; J. Ethnopharmacol. 36, 239 (1992) | (2) T.B. Ng et al.; Life Sci. 66, 709 (2000) | (3) W. He, et al.; Cell. Mol. Neurobiol. 33, 715 (2013) | (4) P. Caboni, et al.; Pest. Manag. Sci. 71, 1099 (2015) | (5) Y. Bai, et al.; J. Funct. Foods 20, 453 (2016) | (6) D. Li & L. Wu; Exp. Ther. Med. 14, 874 (2017) | (7) X. Lin, et al.; Phytomed. 105, 154364 (2022) | (8) X. Gao, et al.; Eur. J. Pharmacol. 960, 176147 (2023)





