Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Innaxon
LPS from E. coli O55:B5 (S-form) TLRpure Sterile Solution

| Product Details | |
|---|---|
| Synonyms | Lipopolysaccharide (LPS) from E. coli O55:B5, S-type (smooth/wild-type) LPS |
| Product Type | Chemical |
| Properties | |
| Source/Host Chemicals | Isolated and purified from E. coli O55:B5. |
| Purity Chemicals | Ultrapure. No detectable DNA, RNA and protein traces. |
| Formulation | Liquid. Colourless clear aqueous solution. |
| Concentration | 1mg/ml stabilised in sterile, double-distilled water (ddWater), without any additives. |
| Biological Activity |
Optimal concentration is dependent upon cell type, species, desired activation and analysis: 0.01-1.0μg/ml. Does not activate any TLR other than TLR4 as tested up to 50μg/ml in relevant cellular systems (macrophages). |
| Declaration | Manufactured by Innaxon. |
| Other Product Data |
TLRpure™: • Qualified Purity & Activity • High potency TLR4-specific Ligands • Ultrapure (no detectable protein, RNA and DNA) • Tested on TLR4 KO murine macrophages • Standardised Aqueous Sterile Solutions • No purification or hazardous solubilisation • Excellent lot-to-lot consistency |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Handling Advice |
Do not freeze. Prepare diluted LPS working solutions just prior to use, keep sterile. Ready-made solution is cell culture-grade. To yield a 100μg/ml (1,000-100x) stock solution add 100μl of LPS to 900μl endotoxin-free and sterile ddWater (Cat. No.: IAX-900-002), 0.9% NaCl Solution (Cat. No.: IAX-900-003) or PBS (Cat. No.: IAX-900-001) and mix well. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
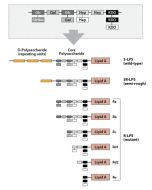
Activation of cells by LPS is mediated by the Toll-like receptor 4 (TLR4). For optimal interaction with LPS, TLR4 requires association with myeloid differentiation protein 2 (MD-2). According to current consensus activation of TLR4 is preceded by the transfer of LPS to membrane-bound (m) or soluble (s) CD14 by LPS-binding protein (LBP). Re-form LPS and lipid A, but not S-form LPS, are capable of inducing TNF-α responses also in the absence of CD14. LPS, synthesized by most wild-type (WT) Gram-negative bacteria (S-form LPS), consists of three regions, the O-polysaccharide chain, which is made up of repeating oligosaccharide units, the core oligosaccharide and the lipid A, which harbors the endotoxic activity of the entire molecule. R-form LPS synthesized by the so-called rough (R) mutants of Gram-negative bacteria lacks the O-specific chain. Furthermore, the core-oligosaccharide may be present in different degrees of completion, depending on the class (Ra to Re) to which the mutant belongs. LPS are amphipathic molecules whose hydrophobicity decreases with increasing length of the sugar part. Based upon these differences, S- and R-form LPS show marked differences in the kinetics of their blood clearance and cellular uptake as well as in the ability to induce oxidative burst in human granulocytes and to activate the host complement system.
- Attachment to erythrocytes of uniform salt forms of lipopolysaccharides from Salmonella abortus-equi and its inhibition by various animal sera: M.D. Praino, et al.; Immunol. Commun. 8, 85 (1979)
- Preparation and properties of a standardized lipopolysaccharide from salmonella abortus equi: C. Galanos, et al.; Zentralbl. Bakteriol. Orig. A. 243, 226 (1979)
- Large-scale fractionation of S-form lipopolysaccharide from Salmonella abortus equi. Chemical and serological characterization of the fractions: C. Galanos, et al.; J. Chromatogr. 440, 397 (1988)
- Immunoblot analysis of the R-form lipopolysaccharide from Salmonella S forms: S. Schlecht, et al.; Zentralbl. Bakteriol. 277, 288 (1992)
- Differential clearance and induction of host responses by various administered or released lipopolysaccharides: R. Hasunuma, et al.; J. Endotoxin Res. 7, 421 (2001)
- The Novel Toll-Like Receptor 2 Agonist SUP3 Enhances Antigen Presentation and T Cell Activation by Dendritic Cells: X. Guo, et al.; Front. Immunol. 8, 158 (2017)
- Modulation of the innate immune-related genes expression in H9N2 avian influenza virus-infected chicken macrophage-like cells (HD11) in response to Escherichia coli LPS stimulation: X. Qi, et al.; Res. Vet. Sci. 111, 36 (2017)
- Marginal zone formation requires ACKR3 expression on B cells: E. Radice, et al.; Cell Rep. 32, 107951 (2020)
- Post-injury immunosuppression and secondary infections are caused by an AIM2 inflammasome-driven signaling cascade: S. Roth, et al.; Immunity 54, 648 (2021)
- A macrophage-T cell coculture model for severe tissue injury-induced T cell death: J. Zhu, et al.; Cell Protocol 2, 100983 (2021)