Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
RevMab
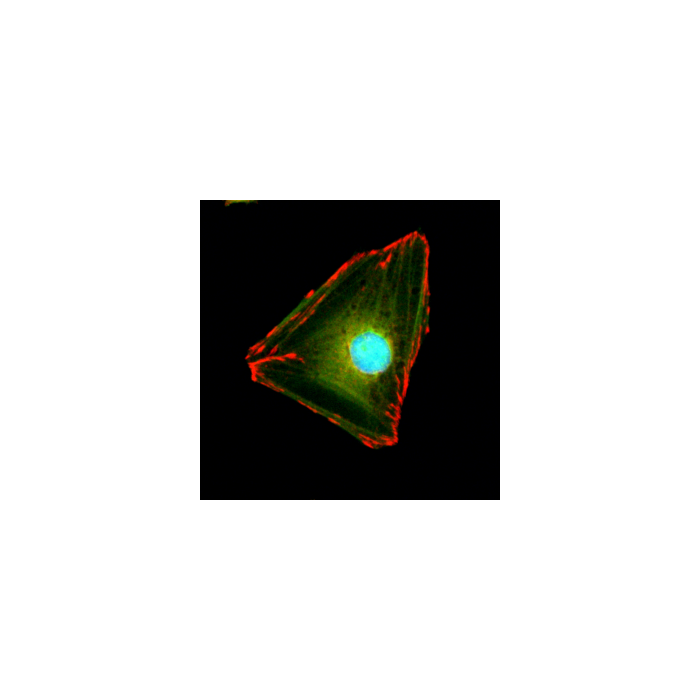
anti-Paxillin (human), Rabbit Monoclonal (RM256)
| Product Details | |
|---|---|
| Synonyms | PXN |
| Product Type | Recombinant Antibody |
| Properties | |
| Clone | RM256 |
| Isotype | Rabbit IgG |
| Source/Host | Rabbit |
| Immunogen/Antigen | A peptide corresponding to the N-terminus of human Paxillin. |
| Application |
Immunocytochemistry (ICC): 1:1000-1:2000 dilution |
| Crossreactivity | Human |
| Specificity |
This antibody reacts to human Paxillin. This antibody may also react to mouse or rat Paxillin, as predicted by immunogen homology. |
| Purity | Protein A purified. |
| Purity Detail | Protein A affinity purified from an animal origin-free culture supernatant. |
| Concentration | N/A |
| Formulation | Liquid. 50% Glycerol/PBS with 1% BSA and 0.09% sodium azide. |
| Isotype Negative Control | |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| Accession Number | P49023 |
| Declaration | Manufactured by RevMab Biosciences. |
| Shipping and Handling | |
| Shipping | BLUE ICE |
| Long Term Storage | -20°C |
| Handling Advice | Avoid freeze/thaw cycles. |
| Use/Stability | Stable for at least 1 year after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Paxillin is a signal transduction adaptor or scaffold protein. This class of molecule provides a structural framework to facilitate the concurrent binding of protein components in particular signalling pathways, thereby promoting efficient and sequential activation of the individual components, as well as controlling crosstalk between pathways and retaining signalling specificity. The C-terminal region of paxillin contains four LIM domains that target paxillin to focal adhesions. It is presumed through a direct association with the cytoplasmic tail of beta-integrin. The N-terminal region of paxillin is rich in protein–protein interaction sites. The proteins that bind to paxillin are diverse and include protein tyrosine kinases, such as Src and focal adhesion kinase (FAK), structural proteins, such as vinculin and actopaxin, and regulators of actin organization, such as COOL/PIX and PKL/GIT. Paxillin is tyrosine-phosphorylated by FAK and Src upon integrin engagement or growth factor stimulation, creating binding sites for the adapter protein Crk. Paxillin is expressed at focal adhesions of non-striated cells and at costameres of striated muscle cells, and it functions to adhere cells to the extracellular matrix. Paxillin has been shown to have a clinically-significant role in patients with several cancer types. Enhanced expression of paxillin has been detected in premalignant areas of hyperplasia, squamous metaplasia and goblet cell metaplasia, as well as dysplastic lesions and carcinoma in high-risk patients with lung adenocarcinoma. Mutations in PXN have been associated with enhanced tumor growth, cell proliferation and invasion in lung cancer tissues.





