Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
RevMab
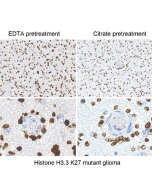
Histone H3 Mutation Antibody Panel (K4M, K9M, K27M, K36M)

| Product Details | |
|---|---|
| Product Type | Set |
| Properties | |
| Application Set | Other |
| Specificity |
Histone H3 Antibody Panel reacts to Histone H3 K4M, K9M, K27M, and K36M mutants. No cross-reactivity with wild-type Histone H3 has been shown. Western Blot: 1:200-1:2000 |
| Crossreactivity |
Human All Vertebrates |
| Sample Type | Serum |
| Kit Contains |
Contains 25µl of each antibody in this Antibody Panel Set |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| Declaration | Manufactured by RevMab Biosciences. |
| Shipping and Handling | |
| Shipping | BLUE ICE |
| Long Term Storage | -20°C |
| Handling Advice | Avoid freeze/thaw cycles. |
| Use/Stability | Stable for at least 1 year after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Histone H3 is one of the DNA-binding proteins found in the chromatin of all eukaryotic cells. H3 along with four core histone proteins binds to DNA forming the structure of the nucleosome. Histones play a central role in transcription regulation, DNA repair, DNA replication and chromosomal stability. Histone H3 has three main variants, H3.1 and H3.2, which are deposited in chromatin only during DNA replication and H3.3, which is replication independent and is found primarily in the regions of active transcription and heterochromatin. Post translationally, histones are modified in a variety of ways to either directly change the chromatin structure or allow for the binding of specific transcription factors. The N-terminal tail of histone H3 protrudes from the globular nucleosome core and can undergo several different types of post-translational modification that influence cellular processes. These modifications include the covalent attachment of methyl or acetyl groups to lysine and arginine amino acids and the phosphorylation of serine or threonine. Histone modifications are one form of epigenetic information that relate closely to gene regulation. Aberrant histone methylation caused by alteration in chromatin-modifying enzymes has long been implicated in cancers. Recently, recurrent histone mutations have been identified in multiple cancers and have been shown to impede histone methylation. All identified histone mutations (including H3K4M, H3K9M, H3K27M, H3K36M, and H3G34V/R/W) result in amino acid substitution at/near a lysine residue that is a target of methylation.