Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
SouthBayBio
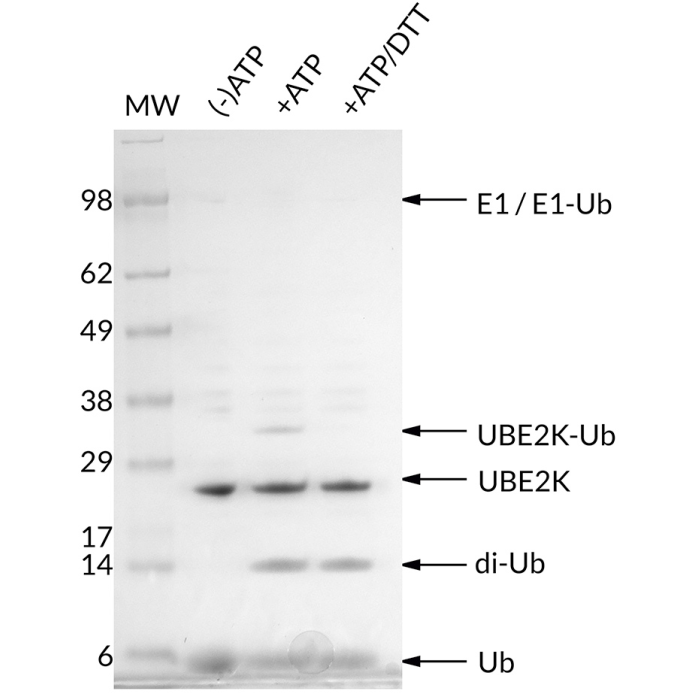
UbcH1 [UBE2K, E2-25K] (human) (rec.) (His)
| Product Details | |
|---|---|
| Synonyms | Ubiquitin-conjugating Enzyme E2; E2-25K; Hip2; HYPG; LIG; UBE2K; UBE2K-25K; Huntingtin-interacting Protein 2 |
| Product Type | Protein |
| Properties | |
| Source/Host | E. coli |
| Sequence | Human UBE2K (aa 1-200) (Accession Nr. P61086) is fused at the N-terminus to a His-tag. |
| Crossreactivity | Human |
| Application | Typical working concentration range is 1-5µM. |
| MW | ~22kDa (SDS-PAGE) |
| Purity | ≥95% (SDS-PAGE) |
| Concentration | Lot dependent. |
| Accession Number | P61086 |
| Formulation | Liquid. In 50mM HEPES pH 7.5, 150mM sodium chloride, 10% glycerol (v/v), 2mM TCEP. |
| Other Product Data |
Click here for a Typical Lot-specific Product Datasheet from the Original Manufacturer |
| Declaration | Manufactured by South Bay Bio. |
| Shipping and Handling | |
| Shipping | DRY ICE |
| Short Term Storage | -80°C |
| Long Term Storage | -80°C |
| Handling Advice | Aliquot to avoid freeze/thaw cycles. |
| Use/Stability | Stable for at least 1 year after receipt when stored at -80°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
UBE2K (UbcH1) is an E2 ubiquitin conjugating enzyme. An E1 activating enzyme is required to attach ubiquitin to UBE2K via an active site cysteine. The mechanism of ubiquitin transfer involves the breaking of a E1-Ub thioester linkage, followed by a reformation of a UBE2K-Ub thioester. UBE2K is capable of synthesizing K48-linked ubiquitin chains and can do so without an E3 ubiquitin ligating enzyme present. UBE2K catalyzes free K48-linked polyubiquitin chains and is known to interact with huntingtin and TRIM6. Although an E3 ligase is not required for chain formation, free UBE2K synthesized K48 polyubiquitin chains have been shown to interact with the E3 TRIM6, leading to activation of IKKε kinase activity and subsequent antiviral activity.





