Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
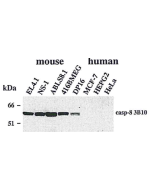
anti-Caspase-8 (human), mAb (C15)

| Product Details | |
|---|---|
| Synonyms | Apoptotic Cysteine Protease; Apoptotic Protease Mch-5; CAP4; FADD-homologous ICE/ced-3-like Protease |
| Product Type | Monoclonal Antibody |
| Properties | |
| Clone | C15 |
| Isotype | Mouse IgG2b |
| Source/Host | Purified from concentrated hybridoma tissue culture supernatant. |
| Immunogen/Antigen | Recombinant human caspase-8 (aa 181-478). |
| Application |
Western Blot: (1μg/ml) |
| Crossreactivity | Human |
| Specificity |
Recognizes the p18 subunit of human caspase-8. |
| Purity | ≥95% (SDS-PAGE) |
| Purity Detail | Protein G-affinity purified. |
| Concentration | 1mg/ml (Lot dependent 0.5 mg/ml) |
| Formulation | Liquid. In PBS containing 10% glycerol and 0.02% sodium azide. |
| Isotype Negative Control | |
| Shipping and Handling | |
| Shipping | BLUE ICE |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
After opening, prepare aliquots and store at -20°C. Avoid freeze/thaw cycles. |
| Use/Stability | Stable for at least 1 year after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Procaspase-8 belongs to the family of caspases. Binding of FasL to Fas leads to formation of a receptor complex at the cellular membrane, which was named DISC. The DISC consists of oligomerized receptors, the DD-containing adaptor molecule FADD, procaspase-8, procaspase-10 and c-FLIP. The DISC structure provides a platform for the oligomerization of procaspase-8 that allows two procaspase-8 homodimers to be in the close proximity leading to the initial activation of procaspase-8. At the first cleavage step, the N-terminal p43/p41 and the C-terminal p30 cleavage products are generated. Importantly, these cleavage products already possess catalytic activity. At the second cleavage step, p43/p41 and p30 are processed to p10 and p18, respectively, which leads to the generation of the active caspase-8 heterotetramer (p18/p10)2.
- FLICE is predominantly expressed as two functionally active isoforms, caspase-8/a and caspase-8/b: C. Scaffidi, et al.; J. Biol. Chem. 272, 26953 (1997)
- Bcl-xL Acts Downstream of Caspase-8 Activation by the CD95 Death-inducing Signaling Complex: J.P. Medema, et al.; J. Biol. Chem. 273, 3388 (1998)
- Two CD95 (APO-1/Fas) signaling pathways: C. Scaffidi, et al.; EMBO J. 17, 1675 (1998)
- Liposomal ET-18-OCH3 Induces Cytochrome c-Mediated Apoptosis Independently of CD95 (APO-1/Fas) Signaling: O. Cuvillier, et al. Blood 94, 3583 (1999)
- FADD/MORT1 and Caspase-8 Are Recruited to TRAIL Receptors 1 and 2 and Are Essential for Apoptosis Mediated by TRAIL Receptor 2: M.R. Sprick, et al.; Immunity 12, 599 (2000)
- Involvement of caspases and of mitochondria in Fas ligation-induced eosinophil apoptosis: modulation by interleukin-5 and interferon-gamma: S. Letuve, et al.; J. Leukoc. Biol. 70, 767 (2001)
- Caspase-10 is recruited to and activated at the native TRAIL and CD95 death-inducing signalling complexes in a FADD-dependent manner but can not functionally substitute caspase-8: M.R. Sprick, et al.; EMBO J. 21, 4520 (2002)
- Proteasome inhibition results in TRAIL sensitization of primary keratinocytes by removing the resistance-mediating block of effector caspase maturation: M. Leverkus, et al.; Mol. Cell. Biol. 23, 777 (2003)
- Lack of Proapoptotic Activity of Soluble CD95 Ligand Is Due to Its Failure to Induce CD95 Oligomers: S. Jang, et al.; J. Int. Cyt. Res. 23, 441 (2003)
- Suramin inhibits death receptor-induced apoptosis in vitro and fulminant apoptotic liver damage in mice: S.T. Eichhorst, et al.; Nature Med. 10, 602 (2004)
- Caspase-2 is activated at the CD95 death-inducing signaling complex in the course of CD95-induced apoptosis: I.N. Lavrik, et al.; Blood 108, 559 (2006)
- The c-FLIP-NH2 terminus (p22-FLIP) induces NF-kappaB activation: A. Golks, et al.; J. Exp. Med. 203, 1295 (2006)
- The role of CAP3 in CD95 signaling: new insights into the mechanism of procaspase-8 activation: A. Golks, et al.; Cell Death Diff. 13, 489 (2006)
- Retinoic acid induces caspase-8 transcription via phospho-CREB and increases apoptotic responses to death stimuli in neuroblastoma cells: M. Jiang, et al.; Biochim. Biophys. Acta 1783, 1055 (2008)
- CD95 Stimulation Results in the Formation of a Novel Death Effector Domain Protein-containing Complex: I.N. Lavrik, et al.; J. Biol. Chem. 283, 26401 (2008)
- Critical Role for Caspase-8 in Epidermal Growth Factor Signaling: D. Finaly, et al.; Cancer Res. 69, 5023 (2009)
- A New C-terminal Cleavage Product of Procaspase-8, p30, Defines an Alternative Pathway of Procaspase-8 Activation: J.C. Hoffmann, et al.; Mol. Cell Biol. 29, 4431 (2009)
- 2D-gel Electrophoresis As a Tool to Investigate the Composition of CD95 DISC: D. Riess & I. Lavrik; Acta Naturae 2, 96 (2010)
- Novel HTS Strategy Identifies TRAIL-Sensitizing Compounds Acting Specifically Through the Caspase-8 Apoptotic Axis: D. Finlay, et al.; PLoS One 5, e13375 (2010)
- Stoichiometry of the CD95 Death-Inducing Signaling Complex: Experimental and Modeling Evidence for a Death Effector Domain Chain Model: K. Schleich, et al.; Mol. Cell 47, 1 (2012)
- Caspase-8 Acts in a Non-enzymatic Role as a Scaffold for Assembly of a Pro-inflammatory “FADDosome” Complex upon TRAIL Stimulation: C.M. Henry & S.J. Martin; Mol. Cell 65, 715 (2017)
- Systemic network analysis identifies XIAP and IκBα as potential drug targets in TRAIL resistant BRAF mutated melanoma: G. Del Mistro, et al.; NPJ Syst. Biol. Appl. 4, 39 (2018)
- TRAIL receptors serve as stress-associated molecular patterns to promote ER-stress-induced inflammation: G.P. Sullivan, et al.; Dev. Cell. 52, 714 (2020)
- Focal adhesion kinase plays a dual role in TRAIL resistance and metastatic outgrowth of malignant melanoma: G. Del Mistro, et al.; Cell Death Dis. 13, 54 (2022)
- Human ZBP1 induces cell death-independent inflammatory signaling via RIPK3 and RIPK1: R. Peng, et al.; EMBO Rep. e55839 (2022)
- cFLIPL acts as a suppressor of TRAIL- and Fas-initiated inflammation by inhibiting assembly of caspase-8/FADD/RIPK1 NF-kB-activating complexes: P. Davidovich, et al.; Cell Rep. 42, 113476 (2023)