Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
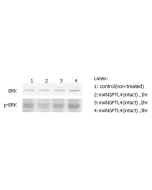
ANGPTL4 (fibrinogen-like domain) (human) (rec.)

| Product Details | |
|---|---|
| Synonyms | Angiopoietin-like Protein 4; FIAF; Fasting-induced Adipose Factor; HFARP; Hepatic Fibrinogen/Angiopoietin-related Protein |
| Product Type | Protein |
| Properties | |
| Source/Host | HEK 293 cells |
| Sequence | Fibrinogen-like domain of human ANGPTL4 (aa 166-406) is fused at the N-terminus to a FLAG®-tag. |
| Crossreactivity | Human |
| MW | ~35kDa (SDS-PAGE) |
| Purity | ≥90% (SDS-PAGE) |
| Endotoxin Content | <0.1EU/μg purified protein (LAL test; Lonza). |
| Concentration | 0.5 mg/ml |
| Formulation | Liquid. 0.2μm-filtered solution in PBS. |
| Other Product Data |
UniProt link Q9BY76: ANGPTL4 (human) [Precursor] FLAG is a registered trademark of Sigma-Aldrich Co. |
| Shipping and Handling | |
| Shipping | BLUE ICE |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
After opening, prepare aliquots and store at -20°C. Avoid freeze/thaw cycles. For maximum product recovery after thawing, centrifuge the vial before opening the cap. |
| Use/Stability |
Stable for at least 6 months after receipt when stored at -20°C. Working aliquots are stable for up to 3 months when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
ANGPTL4 (Angiopoietin-like protein 4) mainly expressed in endothelial cells (hypoxia-induced). Regulates angiogenesis and modulates tumorigenesis and directly regulates lipid, glucose, and energy metabolism. Inhibits proliferation, migration, and tubule formation of endothelial cells and reduces vascular leakage. ANGPTL4 is a protein consisting of an N-terminal coiled-coil domain and a C-terminal fibrinogen-like domain (FLD). Both domains have distinct biological functions. The coiled-coil domain is responsible for the inhibitory effects on lipoprotein lipase (LPL) converting the active form of LPL into an inactive form, and the FLD domain mediates its antiangiogenic functions. The coiled coil and the FLD domains are separated by a short linker that can be cleaved after secretion. ANGPTL4 appears on the cell surface as the full-length form, where it can be released by heparin treatment. ANGPTL4 protein is then proteolytically cleaved by proprotein convertases (PCs), including furin, PC5/6, paired basic amino acid-cleaving enzyme 4, and PC7.
- High Concentrations of Angiopoietin-Like Protein 4 Detected in Serum from Patients with Rheumatoid Arthritis Can Be Explained by Non-Specific Antibody Reactivity: E. Makoveichuk, et al.; PLoS One 12, e0168922 (2017)