Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
Arginase I (human) (rec.) (highly active)
| Product Details | |
|---|---|
| Synonyms | EC 3.5.3.1; ARG1; Arginase 1; Type I Arginase; Liver-type Arginase; L-Arginase |
| Product Type | Protein |
| Properties | |
| Source/Host | E. coli |
| Sequence |
Full length human arginase I. |
| Crossreactivity | Human |
| Biological Activity |
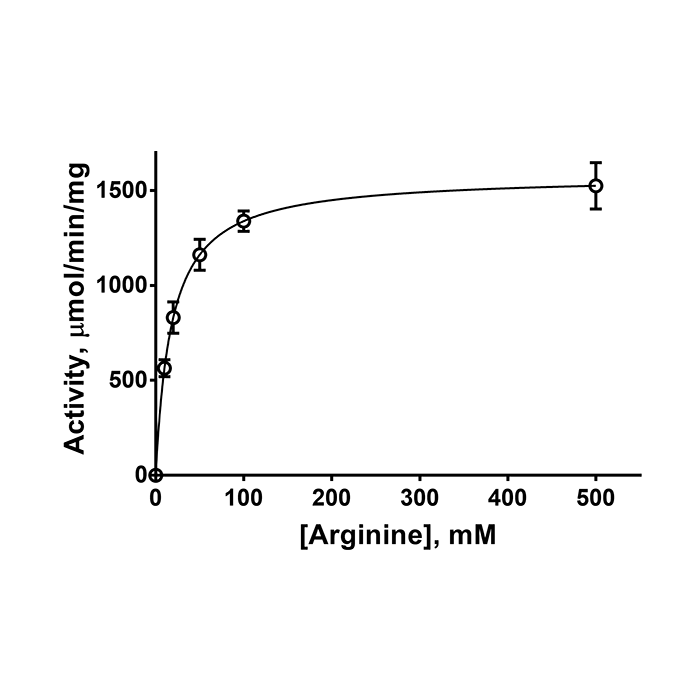
1.6 ±0.2U/µg protein. One unit is defined as the amount of enzyme that converts 1µmol of L-arginine to L-ornithine and urea per min. at 37°C, pH 9.5 (according to protocol from R.T. Schimke, et al.; J. Biol. Chem. 238, 1012 (1963)). |
| MW | 34.7kDa |
| Purity | ≥90% (SDS-PAGE) |
| Concentration | 0.2μg/μl |
| Accession Number | P05089 |
| Formulation | Liquid. In 10mM TRIS-HCl, pH 7.5, containing 1mM β-mercaptoethanol, 1mM MnCl2 and 50% glycerol. |
| Other Product Data |
Uniprot P05089: Arginase 1 (human) |
| Shipping and Handling | |
| Shipping | DRY ICE |
| Short Term Storage | -20°C |
| Long Term Storage | -80°C |
| Handling Advice | Avoid freeze/thaw cycles. |
| Use/Stability | Stable for at least 1 year after receipt when stored at -80°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Arginase catalyzes the hydrolysis of arginine to ornithine and urea. At least two isoforms of mammalian arginase exist (types I and II) which differ in their tissue distribution, subcellular localization, immunologic crossreactivity and physiologic function. The type I isoform is a cytosolic enzyme and expressed predominantly in the liver as a component of the urea cycle. Inherited deficiency of this enzyme results in argininemia, an autosomal recessive disorder characterized by hyperammonemia. Arginase is involved in the nitric oxide (NO) pathway and immune cell arginine metabolism. It is fundamentally involved in cancer, inflammation, infections, fibrotic diseases, neurobiology, pregnancy and immune regulation in general.
- Determination of arginase activity in macrophages: a micromethod: I.M. Corraliza, et al.; J. Immunol. Methods 174, 231 (1994)
- Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells: M. Munder, et al.; J. Immunol. 163, 3771 (1999)
- Implications of the S-shaped domain in the quaternary structure of human arginase: A. Mora, et al.; Biochim. Biophys. Acta 1476, 181 (2000)
- Glu-256 is a main structural determinant for oligomerisation of human arginase I: G. Sabio, et al.; FEBS Letters 501, 161 (2001)
- Crystal structure of human arginase I at 1.29-Å resolution and exploration of inhibition in the immune response: L. Di Costanzo, et al.; PNAS 102, 13058 (2005)