Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
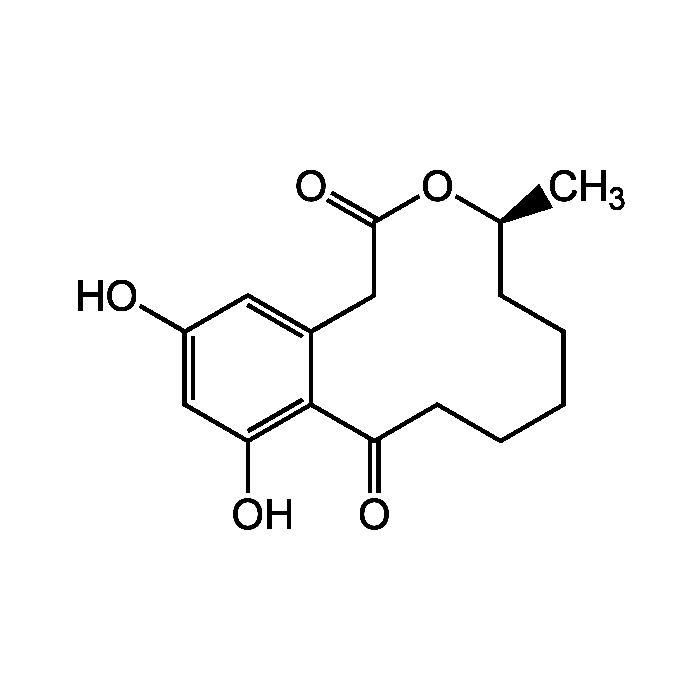
Curvularin
As low as
70
CHF
CHF 70.00
In stock
Only %1 left
AG-CN2-0147-M0011 mgCHF 70.00
AG-CN2-0147-M0055 mgCHF 280.00
| Product Details | |
|---|---|
| Synonyms | (S)-Curvularin; NSC 166071 |
| Product Type | Chemical |
| Properties | |
| Formula |
C16H20O5 |
| MW | 292.3 |
| CAS | 10140-70-2 |
| Source/Host Chemicals | Isolated from Penicillium sp. FKI-1938 |
| Purity Chemicals | ≥95% (HPLC) |
| Appearance | Off-white solid. |
| Solubility | Soluble in ethanol, methanol, DMSO, dioxane or pyridine. Insoluble in water, hexane, benzene or chloroform. |
| InChi Key | VDUIGYAPSXCJFC-JTQLQIEISA-N |
| Smiles | C[C@H]1CCCCCC(=O)C2=C(O)C=C(O)C=C2CC(=O)O1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Keep cool and dry. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Antibiotic [1].
- Antiprotozoal [1].
- Antifungal and phytotoxic compound [1, 6].
- Anti-inflammatory [7].
- TGF-β signaling inhibitor [8]
- Anticancer compound [2, 9].
- Inhibits expression of inducible nitric oxide synthase (iNOS; NOSII) [4, 5].
- Cell division inhibitor [3].
- Acetylcholinesterase (AChE) inhibitor [9].
Product References
- Antimicrobial properties of fungal macrolide antibiotics: V. Betina & D. Micekova; Z. Allg. Mikrobiol. 12, 355 (1972)
- Curvularin from Penicillium baradicum Baghdadi NRRL 3754, and biological effects: R.F. Vesonder, et al.; J. Environ. Sci. Health 11, 289 (1976)
- Structural study of curvularin, a cell division inhibitor: E.L. Ghisalberti, et al.; Austral. J. Chem. 46, 571 (1993)
- Sporogen, S14-95, and S-curvularin, three inhibitors of human inducible nitric-oxide synthase expression isolated from fungi: Y. Yao, et al.; Mol. Pharmacol. 63, 383 (2003)
- Inhibitors of inducible NO synthase expression: total synthesis of (S)-curvularin and its ring homologues: S. Elzner, et al.; ChemMedChem 3, 924 (2008)
- Isolation and Difference in Anti-Staphylococcus aureus Bioactivity of Curvularin Derivates from Fungus Eupenicillium sp.: L.W. Xie, et al.; Appl. Biochem. Biotechnol. 159, 284 (2009)
- The anti-inflammatory fungal compound (S)-curvularin reduces proinflammatory gene expression in an in vivo model of rheumatoid arthritis: N. Schmidt, et al.; J. Pharmacol. Exp. Ther. 343, 106 (2012)
- Inhibition of TGF-β signaling by the fungal lactones (S)-curvularin, dehydrocurvularin, oxacyclododecindione and galiellalactone: K. Rudolph, et al.; Cytokine 61, 285 (2013)
- Metabolite profiling and biological activities of bioactive compounds produced by Chrysosporium lobatum strain BK-3 isolated from Kaziranga National Park, Assam, India: C.G. Kumar, et al.; SpringerPlus 2, 122 (2013)