Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
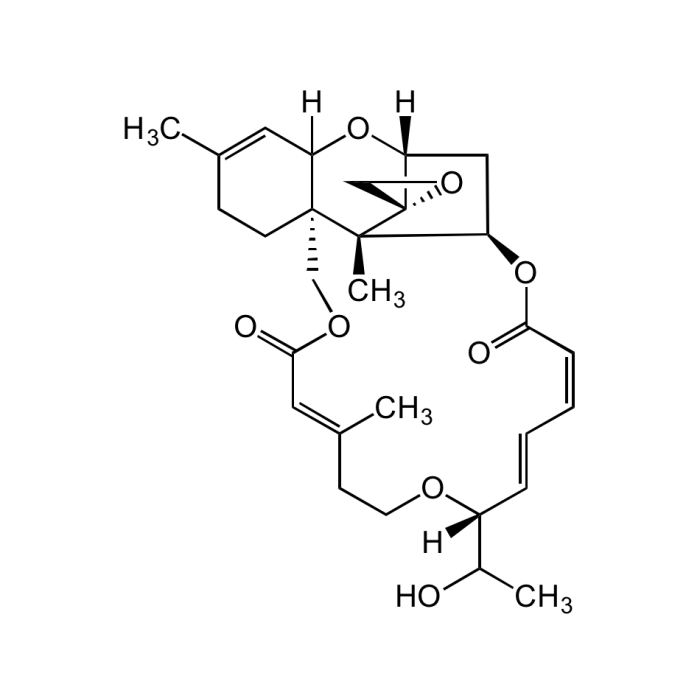
Roridin E
As low as
110
CHF
CHF 110.00
In stock
Only %1 left
AG-CN2-0176-C250250 µgCHF 110.00
AG-CN2-0176-M0011 mgCHF 330.00
| Product Details | |
|---|---|
| Synonyms | Satratoxin D; 2',3'-Didehydro-7'-deoxo-2'-deoxy-7'-(1-hydroxy-ethyl)verrucarin A |
| Product Type | Chemical |
| Properties | |
| Formula |
C29H38O5 |
| MW | 514.6 |
| CAS | 16891-85-3 |
| RTECS | YX9821500 |
| Source/Host Chemicals | Isolated from fungus Trichoderma sp. |
| Purity Chemicals | ≥95% (HPLC) |
| Appearance | Off-white solid. |
| Solubility | Soluble in ethanol, methanol or DMSO (all 1mg/ml). |
| InChi Key | KEEQQEKLEZRLDS-ALFAKORJSA-N |
| Smiles | [H]C12[C@@]3([C@]4(C)[C@]5(CO5)[C@](C[C@H]4OC(/C=C\C=C\[C@](OCC/C(C)=C/C(OC3)=O)([H])C(O)C)=O)([H])O2)CCC(C)=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Keep cool and dry. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Mycotoxin. Implicated in human and animal toxicosis.
- Potent cytotoxic and antiproliferative agent against cancer cell lines.
- Potent antimalarial agent.
- Antifungal, antibiotic, phototoxic and cytostatic agent.
- Antiviral against arenavirus Junin (JUNV).
Product References
- Structure of the antibiotic Roridin E: P. Traxler, et al.; Helv. Chim. Acta 53, 2071 (1970)
- Effects of macrocyclic trichothecene mycotoxins on the murine immune system: B.J. Hughes, et al.; Arch. Environ. Contam. Toxicol. 18, 388 (1989)
- Antimalarial activity of macrocyclic trichothecenes isolated from the fungus Myrothecium verrucaria: M. Isaka, et al.; J. Nat. Prod. 62, 329 (1999)
- Phytotoxicity and mammalian cytotoxicity of macrocyclic trichothecene mycotoxins from Myrothecium verrucaria: H.K. Abbas, et al.; Phytochem. 59, 309 (2002)
- Evaluation of the antiviral activity against Junin virus of macrocyclic trichothecenes produced by the hypocrealean epibiont of Baccharis coridifolia: C.C. Garcia, et al.; Planta Med. 68, 209 (2002)
- 12'-Hydroxyl group remarkably reduces Roridin E cytotoxicity: T. Oda, et al.; Mycosciences 51, 317 (2010)
- Isolation and characterization of roridin E: C.D. Ridge, et al.; MRC 55, 337 (2017)
- Preparative separation and purification of trichothecene mycotoxins from the marine fungus Fusarium sp. LS68 by high-speed countercurrent chromatography in stepwise elution mode: Y. Liu, et al.; Mar. Drugs 16, 73 (2018)