Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
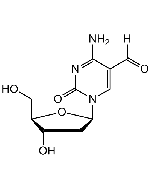
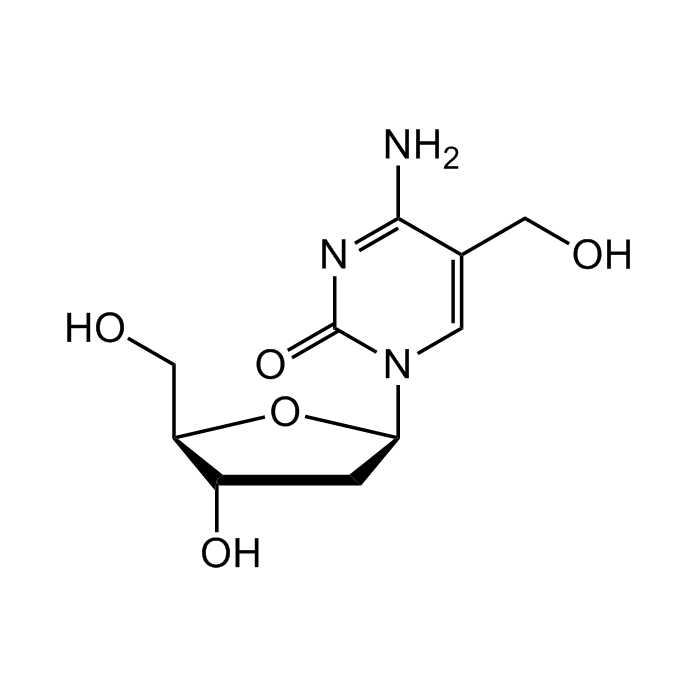
5-(Hydroxymethyl)-2'-deoxycytidine
As low as
40
CHF
CHF 40.00
In stock
Only %1 left
AG-CR1-3532-M0011 mgCHF 40.00
AG-CR1-3532-M0055 mgCHF 140.00
| Product Details | |
|---|---|
| Synonyms | 5 hmdC; 2'-Deoxy-5-(hydroxymethyl)-cytidine |
| Product Type | Chemical |
| Properties | |
| Formula |
C10H15N3O5 |
| MW | 257.2 |
| CAS | 7226-77-9 |
| Purity Chemicals | ≥98% |
| Appearance | White solid. |
| Solubility | Soluble in DMSO (10mg/ml) or water (5mg/ml). |
| Identity | Determined by 1H-NMR. |
| InChi Key | HMUOMFLFUUHUPE-XLPZGREQSA-N |
| Smiles | O[C@H]1C[C@H](N2C=C(CO)C(N)=NC2=O)O[C@@H]1CO |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Hygroscopic. Keep cool and dry. Protect from moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Epigenetic base.
- A modified pyrimidine that is capable of producing interstrand cross-links in double-stranded DNA and has been used to quantify DNA hydroxymethylation levels in biological samples.
- Enriched in the brain, suggesting a role in epigenetic control of neuronal function.
- Used in epigenetic research and important for cancer research.
- Potent HIV-1 reverse transcriptase activity inhibitor.
- Can be used as building block in nucleoside and nucleotide chemistry.
Product References
- Interstrand cross-link formation in duplex and triplex DNA by modified pyrimidines: X. Peng, et al.; JACS 130, 10299 (2008)
- The nuclear DNA base, 5-hydroxymethylcytosine is present in brain and enriched in Purkinje neurons: S. Kriaucionis & N. Heintz; Science 324, 929 (2009)
- 5-Modified-2'-dU and 2'-dC as mutagenic anti HIV-1 proliferation agents: synthesis and activity: Y. El Sadafi, et al.; J. Med. Chem. 53, 1534 (2010)
- Determination of genomic 5-hydroxymethyl-2'-deoxycytidine in human DNA by capillary electrophoresis with laser induced fluorescence: A.M. Krais, et al.; Epigenetics 6, 560 (2011)
- Detection of oxidation products of 5-methyl-2'-deoxycytidine in Arabidopsis DNA: S. Liu, et al.; PLoS One 8, e84620 (2013)
- Synthesis of a DNA promoter segment containing all four epigenetic nucleosides: 5-Methyl-, 5-hydroxymethyl-, 5-formyl-, and 5-carboxy-2'-deoxycytidine: A.S. Schroeder, et al.; Angew. Chem. Int. Ed. 53, 315 (2014)
- Hydroxyl-radical-induced oxidation of 5-methylcytosine in isolated and cellular DNA: G.S. Madugundu, et al.; Nucleic Acids Res. 42, 7450 (2014)
- CDA directs metabolism of epigenetic nucleosides revealing a therapeutic window in cancer; M. Zauri, et al.; Nature 524, 114 (2015)