Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
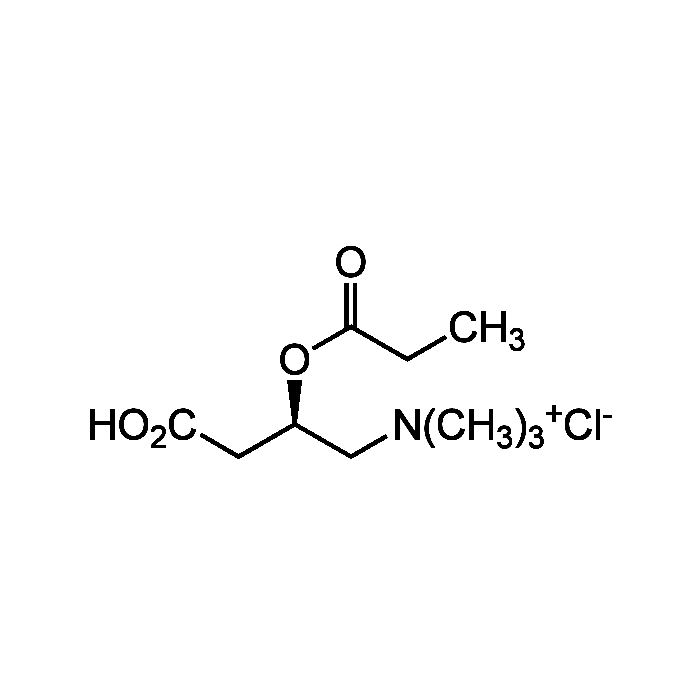
Propionyl-L-carnitine . hydrochloride
As low as
80
CHF
CHF 80.00
In stock
Only %1 left
AG-CR1-3595-M02525 mgCHF 80.00
AG-CR1-3595-M100100 mgCHF 150.00
AG-CR1-3595-M500500 mgCHF 320.00
| Product Details | |
|---|---|
| Synonyms | PLC |
| Product Type | Chemical |
| Properties | |
| Formula |
C10H19NO4 . HCl |
| MW | 217.3 . 36.5 |
| CAS | 119793-66-7 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥98% (NMR) |
| Appearance | White to off-white solid. |
| Solubility | Soluble in water, methanol, ethanol, DMSO or dimethyl formamide. |
| InChi Key | KTFMPDDJYRFWQE-DDWIOCJRSA-N |
| Smiles | CC[C@@H](CC(O)=O)OC(=O)CC |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Hygroscopic. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Naturally occurring carnitine derivative formed by carnitine acetyltransferase during β-oxidation of uneven chain fatty acids, with high affinity for muscular carnitine transferase. Increases cellular carnitine content, allowing free fatty acid transport into the mitochondria [1, 5].
- Stimulates energy production in ischaemic muscles by increasing citric acid cycle flux and stimulating pyruvate dehydrogenase activity [1, 2].
- Important for mitochondrial metabolism and energy regulation. Regulates the metabolism of both carbohydrates and lipids, leading to an increase of ATP generation [9, 11].
- Selectively inhibits in vitro and ex vivo platelet-activating factor (PAF) synthesis from human neutrophils [3].
- Antioxidant. Shows free radical scavenging activity [4].
- Decreases the expression of inducible nitric oxide synthase (iNOS/NOS II) and NADPH-oxidase 4-mediated reactive oxygen species production in human umbilical vascular endothelial cells [7].
- Shows beneficial cardiovascular effects. Improves body weight, food intake, adiposity and insulin resistance in Type 2 diabetes [5, 6, 8-10].
- Stimulates endothelial nitric oxide (eNOS/NOS III) and increased NO production, via AMPK/Src-mediated signaling that leads to activation of PI3 kinase and Akt [6, 12].
Product References
- Changes in skeletal muscle histology and metabolism in patients undergoing exercise deconditioning: effect of propionyl-L-carnitine: G. Brevetti, et al.; Muscle Nerve 20, 1115 (1997)
- Propionyl-L-carnitine: L.R. Wiseman & R.N. Brogden; Drugs Aging 12, 243 (1998) (Review)
- Inhibition of platelet-activating factor synthesis in human neutrophils and platelets by propionyl-L-carnitine: M. Triggiani, et al.; Biochem. Pharmacol. 58, 1341 (1999)
- L-propionyl-carnitine as superoxide scavenger, antioxidant, and DNA cleavage protector: A. Vanella, et al.; Cell Biol. Toxicol. 16, 99 (2000)
- Therapeutic effects of L-carnitine and propionyl-L-carnitine on cardiovascular diseases: a review: R. Ferrari, et al.; Ann. N. Y. Acad. Sci. 1033, 79 (2004) (Review)
- Oral supplementation of propionyl-l-carnitine reduces body weight and hyperinsulinaemia in obese Zucker rats: C. Mingorance, et al.; Br. J. Nutr. 102, 1145 (2009)
- Propionyl-L-carnitine improves postischemic blood flow recovery and arteriogenetic revascularization and reduces endothelial NADPH-oxidase 4-mediated superoxide production: M.A. Stasi, et al.; Arterioscler. Thromb. Vasc. Biol. 30, 426 (2010)
- Critical update for the clinical use of L-carnitine analogs in cardiometabolic disorders: C. Mingorance, et al.; Vasc. Health Risk Manag. 7, 169 (2011)
- Pharmacological effects and clinical applications of propionyl-L-carnitine: C. Mingorance, et al.; Nutr. Rev. 69, 279 (2011) (Review)
- Propionyl-L-carnitine corrects metabolic and cardiovascular alterations in diet-induced obese mice and improves liver respiratory chain activity: C. Mingorance, et al.; PLoS One 7, e34268 (2012)
- Translating the basic knowledge of mitochondrial functions to metabolic therapy: role of L-carnitine: S.M. Marcovina, et al.; Transl. Res. 161, 73 (2013)
- Propionyl-L-carnitine induces eNOS activation and nitric oxide synthesis in endothelial cells via PI3 and Akt kinases: W.H. Ning & K. Zhao; Vascul. Pharmacol. 59, 76 (2013)