Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
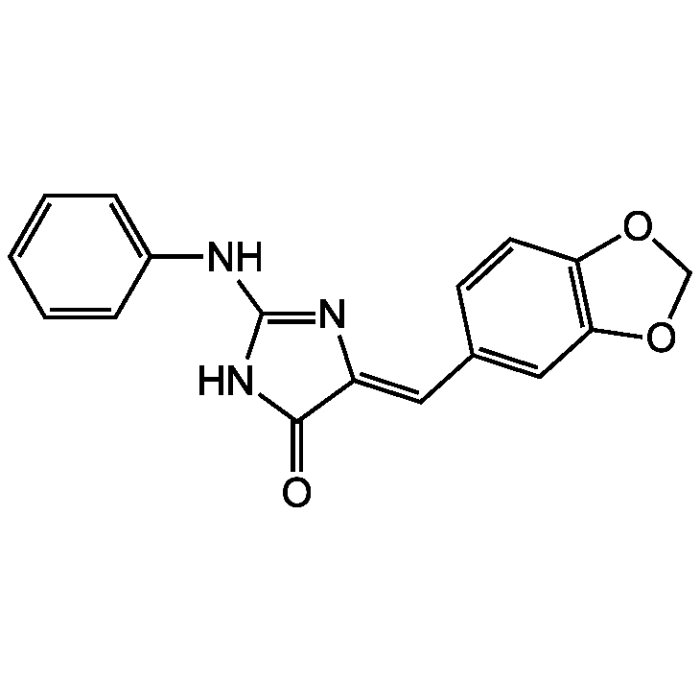
Leucettine L41
As low as
70
CHF
CHF 70.00
In stock
Only %1 left
AG-MR-C0023-M0011 mgCHF 70.00
AG-MR-C0023-M0055 mgCHF 280.00
AG-MR-C0023-M02525 mgCHF 980.00
| Product Details | |
|---|---|
| Synonyms | (5Z)-5-(1,3-Benzodioxol-5-yl)methylene-2-phenylamino-3,5-dihydro-4H-imidazol-4one |
| Product Type | Chemical |
| Properties | |
| Formula |
C17H13N3O3 |
| MW | 307.3 |
| CAS | 1112978-84-3 |
| Purity Chemicals | ≥98% (NMR) |
| Appearance | Beige solid. |
| Solubility | Soluble in DMSO or ethanol. |
| Reconstitution | Stock solutions can be made up to 10mM in DMSO. |
| Identity | Determined by 1H-NMR. |
| InChi Key | PGPHHJBZEGSUNE-JYRVWZFOSA-N |
| Smiles | O=C1NC(NC2=CC=CC=C2)=N\C1=C/C1=CC2=C(OCO2)C=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Keep cool and dry. Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Potent inhibitor of DYRKs (DYRK1A (IC50=40nM); DYRK2 (IC50=35nM)) and CLKs (CLK1, CLK3). Interacts also with GSK-3α/β, CK2 and PIM1.
- Interacts with the complex PIKfyve/Vac14/Fig4.
- Modulates alternative splicing of pre-mRNA in various cellular systems.
- Neuroprotective against APP-induced cell death.
- Tools to study and modulate pre-RNA splicing. Potential compound for Alzheimer's disease and diseases involving abnormal pre-mRNA splicing.
- Potential compound to study Down syndrome (inhibition of DYRK1A).
- Neuroprotective against glutamate-induced cell death.
- Autophagy activator.
Product References
- Leucettines, a class of potent inhibitors of cdc2-like kinases and dual specificity, tyrosine phosphorylation regulated kinases derived from the marine sponge Leucettamine B: modulation of alternative pre-RNA splicing: M. Debdab, et al.; J. Med. Chem. 54, 4172 (2011)
- Selectivity, cocrystal structures, and neuroprotective properties of leucettines, a family of protein kinase inhibitors derived from the marine sponge alkaloid leucettamine B: T. Tahtouh, et al.; J. Med. Chem. 55, 9312 (2012)
- Leucettines, a family of pharmacological inhibitors of DYRKs & CLKs kinases derived from the marine sponge Leucettamine B: T. Tahtouh, et al.; Planta Med. 78, (2012)
- Chemical synthesis and biological validation of immobilized protein kinase inhibitory Leucettines: G. Burgy, et al.; Eur. J. Med. Chem. 62, 728 (2013)
- Cdc-like/dual-specificity tyrosine phosphorylation-regulated kinases inhibitor leucettine L41 induces mTOR-dependent autophagy: implication for Alzheimer's disease: X. Fant, et al.; Mol. Pharmacol. 85, 441 (2014)
- Marine-Derived 2-Aminoimidazolone Alkaloids. Leucettamine B-Related Polyandrocarpamines Inhibit Mammalian and Protozoan DYRK & CLK Kinases: N. Loaec, et al.; Mar. Drugs 15, 316 (2017) (Review)
- Chemically defined and growth-factor-free culture system for the expansion and derivation of human pluripotent stem cells: S. Yasuda, et al.; Nature Biomed. Eng. 2, 173 (2018)
- Correction of cognitive deficits in mouse models of Down syndrome by a pharmacological inhibitor of DYRK1A: T.L. Nguyen, et al.; Dis. Model Mech. 11, dmm035634 (2018)
- Combined Inhibition of DYRK1A, SMAD, and Trithorax Pathways Synergizes to Induce Robust Replication in Adult Human Beta Cells: P. Wang, et al.; Cell Metab. 29, 638 (2019)
- Comparative Efficacy and Selectivity of Pharmacological Inhibitors of DYRK and CLK Protein Kinases: M.F. Lindberg, et al.; J. Med. Chem. 66, 4106 (2023)
- Leucettinibs, a Class of DYRK/CLK Kinase Inhibitors Inspired by the Marine Sponge Natural Product Leucettamine B: E. Deau, et al.; J. Med. Chem. 66, 10694 (2023)
- Chemical, Biochemical, Cellular, and Physiological Characterization of Leucettinib-21, a Down Syndrome and Alzheimer’s Disease Drug Candidate: M.F. Lindberg, et al.; J. Med. Chem. 66, 15648 (2023)










