Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
BellBrook
WRN Helicase (human) (rec.) (His) (active) (1µg)

| Product Details | |
|---|---|
| Synonyms | Bifunctional 3'-5' Exonuclease/ATP-dependent Helicase WRN; RecQ Protein-like 2; RECQ3; RECQL2; WRN RecQ-like Helicase |
| Product Type | Protein |
| Properties | |
| Source/Host | Insect cells |
| Sequence |
Recombinant human WRN Helicase enzyme (aa500-946) fused to a N-terminal His-tag. |
| Crossreactivity | Human |
| Biological Activity |
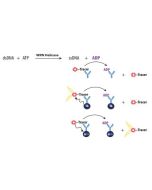
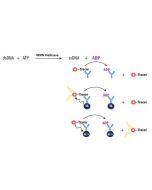
The enzyme has been thoroughly validated with the Transcreener ADP Assay Kit. For specific activity please refer to the Certificate of Analysis for each individual enzyme lot. |
| MW | 51.8kDa |
| Purity | ≥90% (SDS-PAGE) |
| Accession Number | Q14191 |
| Formulation | Liquid. In 50mM Tris-HCl pH 8.0, 500mM NaCl, 20% glycerol buffer, 0.5 mM TCEP. |
| Shipping and Handling | |
| Shipping | DRY ICE |
| Short Term Storage | -20°C |
| Long Term Storage | -80°C |
| Handling Advice | Avoid freeze/thaw cycles. |
| Use/Stability | Stable for at least 6 months after receipt when stored at -80°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
WRN helicase is a multifunctional enzyme that has both magnesium and ATP-dependent DNA-helicase activity and 3'->5' exonuclease activity towards double-stranded DNA with a 5'-overhang. It has no nuclease activity towards single-stranded DNA or blunt-ended double-stranded DNA. It binds preferentially to DNA substrates containing alternate secondary structures, such as replication forks and Holliday junctions. It may play an important role in the dissociation of joint DNA molecules that can arise as products of homologous recombination, at stalled replication forks, or during DNA repair. It alleviates the stalling of DNA polymerases at the site of DNA lesions and is important for genomic integrity. It plays a role in the formation of DNA replication focal centers; stably associates with foci elements generating binding sites for RP-A (By similarity) and plays a role in double-strand break repair after gamma-irradiation.