Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
BioViotica
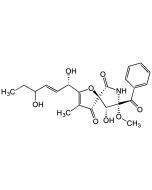
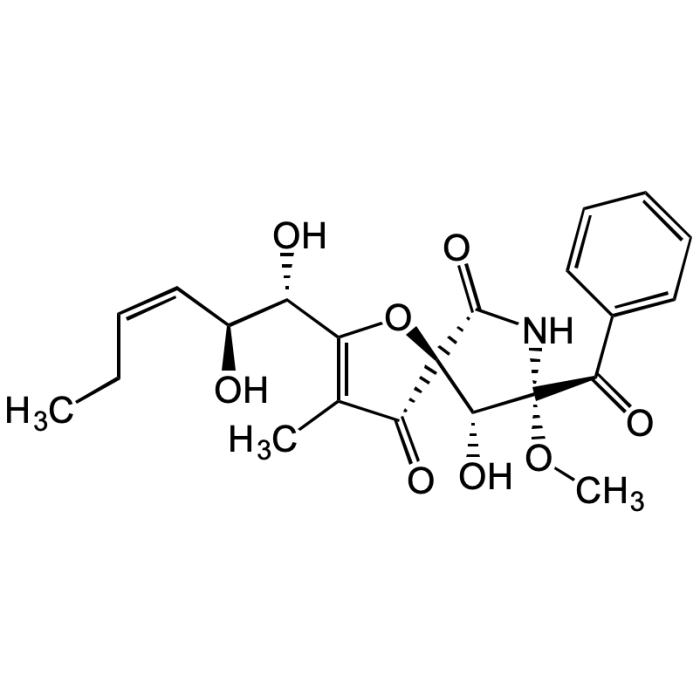
Pseurotin A
As low as
110
CHF
CHF 110.00
In stock
Only %1 left
BVT-0003-M0011 mgCHF 110.00
BVT-0003-M0055 mgCHF 350.00
| Product Details | |
|---|---|
| Synonyms | (5S,8S,9R)-8-Benzoyl-2-((1S,2S,Z)-1,2-dihydroxyhex-3-en-1-yl)-9-hydroxy-8-methoxy-3-methyl-1-oxa-7-azaspiro[4.4]non-2-ene-4,6-dione |
| Product Type | Chemical |
| Properties | |
| Formula |
C22H25NO8 |
| MW | 431.4 |
| CAS | 58523-30-1 |
| Source/Host Chemicals | Isolated from Aspergillus fumigatus. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in methanol or ethyl acetate; poorly soluble in water. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by BioViotica. |
| InChi Key | SLYDIPAXCVVRNY-UOWMTANKSA-N |
| Smiles | CC1=C([C@@H](O)[C@@H](O)/C=C\CC)O[C@@]2([C@@H](O)[C@](OC)(C(C3=CC=CC=C3)=O)NC2=O)C1=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Use/Stability |
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Antibiotic.
- Chitin synthase, monoamine oxidase and IgE production inhibitor.
- Cytotoxic.
- Shows nematicidal and antileishmanial activity.
- Antiproliferative against four different glioma cells. Downregulates the expression of tumour glycolytic enzymes pyruvate kinase M2 (PKM2) and lactate dehydrogenase 5 (LDH5) and upregulates the expression of pyruvate dehydrogenase beta (PDHB), adenosine triphosphate synthase beta (ATPB) and cytochrome C, important regulators for tricarboxylic acid cycle and oxidative phosphorylation.
- Inhibits activation of B-cells and differentiation into the plasma cells
Product References
- Novel neuritogenic activities of pseurotin A and penicillic acid: D. Komagata, et al.; J. Antibiot. 49, 958 (1996)
- Directed biosynthesis of fluorinated pseurotin A, synerazol and gliotoxin: Y. Igarashi, et al.; J. Antibiot. 57, 748 (2004)
- Fumiquinones A and B, nematicidal quinones produced by Aspergillus fumigatus: A. Hayashi, et al.; Biosci. Biotechnol. Biochem. 71, 1697 (2007)
- Identification of a hybrid PKS/NRPS required for Pseurotin A biosynthesis in the human pathogen Aspergillus fumigatus: S. Maiya, et al.; ChemBioChem 8, 1736 (2007)
- Pseurotin A and its analogues as inhibitors of immunoglobulin E production: M. Ishikawa, et al.; Bioorg. Med. Chem. Lett. 19, 1457 (2009)
- Antiparasitic and anticancer constituents of the endophytic fungus Aspergillus sp. strain F1544: S. Martinez-Luis, et al.; Nat. Prod. Commun. 7, 165 (2012)
- Elucidation of Pseurotin Biosynthetic Pathway Points to Trans-Acting C-Methyltransferase: Generation of Chemical Diversity: Y. Tsunematsu, et al.; Angew. Chem. Int. Ed. 53, 8475 (2014)
- Angiogenesis Inhibitors and Anti-Inflammatory Agents from Phoma sp. NTOU4195: M.-S. Lee, et al.; J. Nat. Prod. 79, 2983 (2016)
- Total syntheses of spirocyclic PKS-NRPS-based fungal metabolites: D. Jo & S. Han; Chem. Comm. 54, 6750 (2018)
- Zebrafish-Based Discovery of Antiseizure Compounds from the Red Sea: Pseurotin A2 and Azaspirofuran A: D. Copmans, et al.; ACS Chem. Neurosci. 9, 1652 (2018)
- Antiglioma pseurotin A from marine Bacillus sp. FS8D regulating tumour metabolic enzymes: K. Anjum, et al.; Nat. Prod. Res. 32, 1353 (2018)
- Treatment of epilepsy using pseurotins and azaspirofurans: D. Copmans, et al.; PCT Int. Appl., WO 2019043019 A1 20190307 (2019)
- Natural pseurotins and analogs thereof inhibit activation of B-cells and differentiation into the plasma cells: O. Vasicek, et al.; Phytomedicine in press (2020)