Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
BioViotica
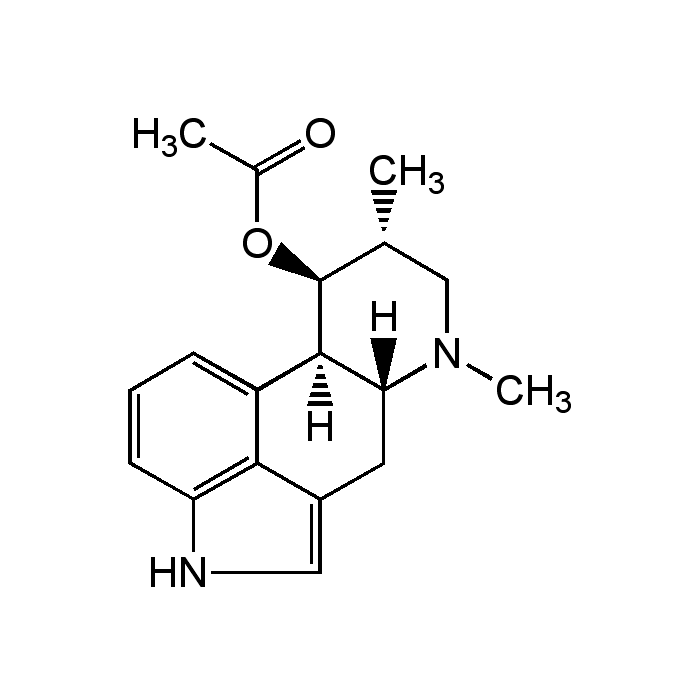
Fumigaclavine A
As low as
170
CHF
CHF 170.00
In stock
Only %1 left
BVT-0090-M0011 mgCHF 170.00
BVT-0090-M0055 mgCHF 505.00
| Product Details | |
|---|---|
| Synonyms | (6aR,9R,10S,10aR)-7,9-Dimethyl-4,6,6a,7,8,9,10,-10a-octahydroindolo[4,3-fg]quinolin-10-yl acetate |
| Product Type | Chemical |
| Properties | |
| Formula |
C18H22N2O2 |
| MW | 298.4 |
| CAS | 6879-59-0 |
| Source/Host Chemicals | Isolated from Aspergillus sp. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Beige to brown solid. |
| Solubility | Soluble in acetone, DMSO or methanol. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by BioViotica. |
| InChi Key | GJSSYQDXZLZOLR-ONUGHKICSA-N |
| Smiles | [H][C@@]12CC3=CNC4=C3C(=CC=C4)[C@@]1([H])[C@@H](OC(C)=O)[C@H](C)CN2C |
| Shipping and Handling | |
| Shipping | BLUE ICE |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Use/Stability |
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Ergot alkaloid.
- Mycotoxin.
- Fumigaclavine A is convertet into fumigaclavine C by the enzyme Reverse Prenyltransferase FgaPT1.
- µ-opioid receptor μOR binding Ki=57μM.
Product References
- Nuclear magnetic resonance spectral analysis of the ergot alkaloids: N.J. Bach, et al.; J. Org. Chem. 39, 1272 (1974)
- Mycotoxins produced by Aspergillusfumigatus isolated from silage: R.J. Cole, et al.; Ann. Nutr. Aliment. 31, 685 (1977)
- Production of fumigaclavine A by Aspergillustamarii Kita: K.K. Janardhanan, et al.; Can. J. Microbiol. 30, 247 (1984)
- Abundant respirable ergot alkaloids from the common airborne fungus Aspergillusfumigatus: D.G. Panaccione& C.M. Coyle; Appl. Environ. Microbiol. 71, 3106 (2005)
- Post-genome research on the biosynthesis of ergot alkaloids: S.M. Li & I.A. Unsold; Planta Med. 72, 117 (2006)
- Reverse prenyltransferase in the biosynthesis of fumigaclavine C in Aspergillus fumigatus: gene expression, purification, and characterization of fumigaclavine C synthase FGAPT1: I.A. Unsöld & S.M. Li; Chembiochem. 7, 158 (2006)
- Clavine Alkaloid biosynthesis by the fungus Penicillium palitans: A.G. Kozlovsky et al.; Appl. Biochem. Microbiol. 45, 182 (2009)
- What makes Aspergillus fumigates a successful pathogen?: A. Abad et al.; Rev. Iberoam. Micol. 27, 155 (2010)
- Production and characterization of antibodies against fumigaclavine A: H. Latif et al.; Mycotoxin Res. 25, 155 (2010)
- Immunochemical analysis of fumigaclavine mycotoxins in respiratory tissues and in blood serum of birds with confirmed aspergillosis: H. Latif, et al.; Mycotoxin Res. 31, 177 (2015)
- Ligand and structure-based approaches for the identification of SIRT1 activators: V.K. Vyas, et al.; Chem. Biol. Interact. 228, 9 (2015)
- Mycotoxin Identification and In Silico Toxicity Assessment Prediction in Atlantic Salmon: J. Tolosa, et al.; Mar. Drugs 18, 629 (2020)
- Identification and quantification of kukoamine A and kukoamine B as novel μ-opioid receptor agonists in potato and other solanaceous plants: J. Saller, et al.; Food Chem. 427, 136637 (2023)