Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
BioViotica
Borrelidin
As low as
95
CHF
CHF 95.00
In stock
Only %1 left
BVT-0098-C500500 µgCHF 95.00
BVT-0098-M0011 mgCHF 145.00
BVT-0098-M0055 mgCHF 480.00
| Product Details | |
|---|---|
| Synonyms | Treponemycin; U 78548; C2989 |
| Product Type | Chemical |
| Properties | |
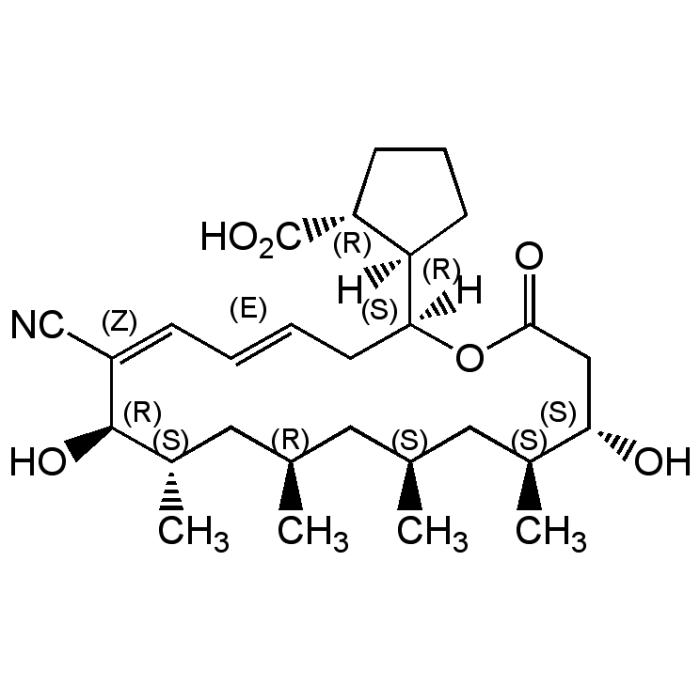
| Formula |
C28H43NO6 |
| MW | 489.6 |
| CAS | 7184-60-3 |
| RTECS | ED8750000 |
| Source/Host Chemicals | Isolated from Streptomyces sp. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in DMSO or methanol. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by BioViotica. |
| InChi Key | OJCKRNPLOZHAOU-TVHKGLGPSA-N |
| Smiles | [H][C@]1(CCC[C@H]1C(O)=O)[C@]1([H])C\C=C\C=C(C#N)/[C@H](O)[C@@H](C)C[C@H](C)C[C@H](C)C[C@H](C)[C@@H](O)CC(=O)O1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light when in solution. |
| Use/Stability |
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Macrolide antibiotic.
- Bacterial, protozoan and mammalian threonyl-tRNA synthetase (THrRS) inhibitor.
- Antiangiogenic (IC50 = 0.8 nM) and anticancer agent.
- Induces the collapse of formed capillary tubes in a dose-dependent fashion. In HUVECs, the capillary tube collapsing activity is mediated by the activation of caspases-3 and -8 and induction of apoptosis.
- Blocks the formation of spontaneous lung metastases of B16-BL6 melanoma cells.
- Cyclin-dependent kinase (CDK) inhibitor.
- Antiviral.
- Potent antimalarial agent (IC50 = 0.97 nM).
- Inhibitor of potato scab disease.
Product References
- Isolation of vivomycin and borrelidin, two antibiotics with anti-viral activity, from a species of Streptomyces (C2989): M. Lumb, et al.; Nature 206, 263 (1965)
- Genetic analysis of mutations causing borrelidin resistance by overproduction of threonyl-transfer ribonucleic acid synthetase: J. Frohler, et al.; J. Bacteriol. 143, 1135 (1980)
- Increased levels of threonyl-tRNA synthetase in a borrelidin-resistant Chinese hamster ovary cell line: J.S. Gantt, et al.; PNAS 78, 5367 (1981)
- Chinese hamster ovary cells resistant to borrelidin overproduce threonyl-tRNA synthetase: S.C. Gerken and S.M. Arfin; J. Biol. Chem. 259, 9202 (1984)
- Isolation of a cDNA clone for human threonyl-tRNA synthetase: amplification of the structural gene in borrelidin-resistant cell lines: K.J. Kontis and S.M. Arfin; Mol. Cell. Biol. 9, 1832 (1989)
- Borrelidin is an angiogenesis inhibitor; disruption of angiogenic capillary vessels in a rat aorta matrix culture model: T. Wakabayashi, et al.; J. Antibiot. 50, 671 (1997)
- Establishment of a quantitative mouse dorsal air sac model and its application to evaluate a new angiogenesis inhibitor: Y. Funahashi, et al.; Oncol. Res. 11, 319 (1999)
- Borrelidin inhibits a cyclin-dependent kinase (CDK), Cdc28/Cln2, of Saccharomyces cerevisiae: E. Tsuchiya, et al.; J. Antibiot. 54, 84 (2001)
- Anti-angiogenesis effects of borrelidin are mediated through distinct pathways: threonyl-tRNA synthetase and caspases are independently involved in suppression of proliferation and induction of apoptosis in endothelial cells: T. Kawamura, et al.; J. Antibiot. 56, 709 (2003)
- Borrelidin induces the transcription of amino acid biosynthetic enzymes via a GCN4-dependent pathway: E.L. Eastwood & S.E. Schaus; Bioorg. Med. Chem. Lett. 13, 2235 (2003)
- A unique hydrophobic cluster near the active site contributes to differences in borrelidin inhibition among threonyl-tRNA synthetases: B. Ruan, et al.; J. Biol. Chem. 280, 571 (2005)
- Borrelidin, a potent antimalarial: stage-specific inhibition profile of synchronized cultures of Plasmodium falciparum: A. Ishiyama, et al.; J. Antibiotics 64, 381 (2011)
- A study on the effects of borrelidin on cardiovascular and intestinal smooth muscles: D. V. Bhikshapathi, et al.; Pharm. Lett. 5, 83 (2013)
- Insights into the preclinical treatment of blood-stage malaria by the antibiotic borrelidin: I. G. Azcarate, et al.; Br. J. Pharmacol. 169, 645 (2013)
- Identification of borrelidin binding site on threonyl-tRNA synthetase: M. Li, et al; Biochem. Biophys. Res. Comm. 451, 485 (2014)
- Analogs of natural aminoacyl-tRNA synthetase inhibitors clear malaria in vivo: E. M. Novoa, et al.; PNAS (USA) 111, E5508 (2014)
- Borrelidin has limited anti-cancer effects in bcl-2 overexpressing breast cancer and leukemia cells and reveals toxicity in non-malignant breast epithelial cells: D. Gafiuc, et al.; J. Appl. Toxicol. 34, 1109 (2014)
- Borrelidin or microorganism which produces it as inhibitor of potato scab disease: Y. Kobayashi, et al.; Jap. Kokai Tokkyo Koho JP 2014224102 (2014)
- Borrelidin Induces the Unfolded Protein Response in Oral Cancer Cells and Chop-Dependent Apoptosis: A. Sidhu, et al.; ACS Med. Chem. Lett. 6, 1122 (2015)
- Effect of borrelidin on hepatocellular carcinoma cells in vitro and in vivo: X. Gao, et al.; RSC Adv. 7, 44401 (2017)
- Liposomal borrelidin for treatment of metastatic breast cancer: M. Jeong, et al.; Drug Deliv. Transl. Res. 8, 1380 (2018)
- Aminoacyl-tRNA synthetase inhibition activates a pathway that branches from the canonical amino acid response in mammalian cells: Y. Kim, et al.; PNAS 117, 8900 (2020)