Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
BioViotica
Deoxynojirimycin
As low as
25
CHF
CHF 25.00
In stock
Only %1 left
BVT-0112-M0011 mgCHF 25.00
BVT-0112-M0055 mgCHF 70.00
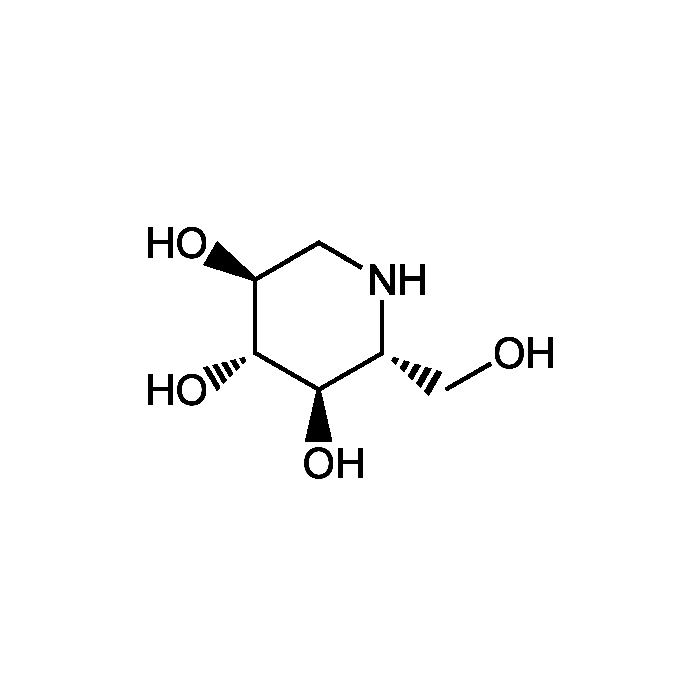
| Product Details | |
|---|---|
| Synonyms | 1,5-Dideoxy-1,5-imino-D-glucitol; Moranoline; DNJ |
| Product Type | Chemical |
| Properties | |
| Formula |
C6H13NO4 |
| MW | 163.2 |
| CAS | 19130-96-2 |
| RTECS | TN4350300 |
| Source/Host Chemicals | Isolated from Streptomyces sp. |
| Purity Chemicals | ≥98% (HPLC, NMR) |
| Appearance | White to off-white solid. |
| Solubility | Soluble in DMSO (10mg/ml) or water (10mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by BioViotica. |
| InChi Key | LXBIFEVIBLOUGU-JGWLITMVSA-N |
| Smiles | OC[C@H]1NC[C@H](O)[C@@H](O)[C@@H]1O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Use/Stability |
Stable for at least 2 years after receipt when stored at -20°C. Store solutions at -20°C in the dark. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Polyhydroxylated piperidine alkaloid.
- α-Glucosidase I and II inhibitor.
- Antidiabetic. Antihyperglycaemic activity.
- Glycosylceramidase inhibitor.
- Animal feedstuff additive.
- Antiviral. Shows anti-HIV activity.
Product References
- Isolation and structure of an unusual cyclic amino alditol from a legume: L.E. Fellows, et al.; Chem. Comm. 22, 977 (1979)
- Glucosidase inhibitors from Bacilli: D.D. Schmidt, et al.; Naturwissenschaften 66, 584 (1979)
- Glucosiddase inhibitors: structures of deoxynojirimycin and castanospermine: A. Hempel, et al.; J. Med. Chem. 36, 4082 (1993)
- Polyhydroxylated alkaloids - natural occurrence and therapeutic applications: A.A. Watson, et al.; Phytochem. 56, 265 (2001)
- Small molecule inhibitors of ER α-glucosidases are active against multiple hemorrhagic fever viruses: J. Chang, et al.; Antiviral Research 98, 432 (2013)
- 1-deoxynojirimycin isolated from a bacillus subtilis stimulates adiponectin and GLUT4 expressions in 3T3-L1 adipocytes: S.-M. Lee, et al.; J. Microb. Biotechn. 23, 637 (2013)
- Novel imino sugar α-glucosidase inhibitors as antiviral compounds: J.D. Howe, et al.; Bioorg. & Med. Chem. 21, 4831 (2013)
- 1-Deoxynojirimycin attenuates high glucose-accelerated senescence in human umbilical vein endothelial cells: E., Shuang, et al.; Exp. Gerontol. 55, 63 (2014)
- 1-Deoxynojirimycin isolated from Bacillus subtilis improves hepatic lipid metabolism and mitochondrial function in high-fat-fed mice: H. Do, et al.; Food Chem. Toxicol. 75, 1, (2015)
- 1-deoxynojirimycin alleviates insulin resistance via activation of insulin signaling PI3K/AKT pathway in skeletal muscle of db/db mice: Q. Liu, et al.; Molecules 20, 21700 (2015)
- Iminosugars as immunomodulating agents: synthesis and biological activities of 1-deoxynojirimycin and related compounds: Q. Li & X.-S. Ye; Isr. J. Chem. 55, 336 (2015)
- Iminosugars: the potential of carbohydrate analogs: N. Asano; Carbohydr. Chem. 2016, 279 (2016)
- 1-Deoxynojirimycin alleviates liver injury and improves hepatic glucose metabolism in db/db mice: Q. Liu, et al.; Molecules 21, 279/1 (2016)
- 1-Deoxynojirimycin: Occurrence, Extraction, Chemistry, Oral Pharmacokinetics, Biological Activities and In Silico Target Fishing: K. Gao, et al.; Molecules 21, 1600 (2016) (Review)
- Protective mechanism of Rice-derived Lipids and Glucosylceramide in an In Vitro Intestinal Tract Model: S. Yamashita, et al.; J. Agric. Food Chem. 69, 10206 (2021)






