Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
BioViotica
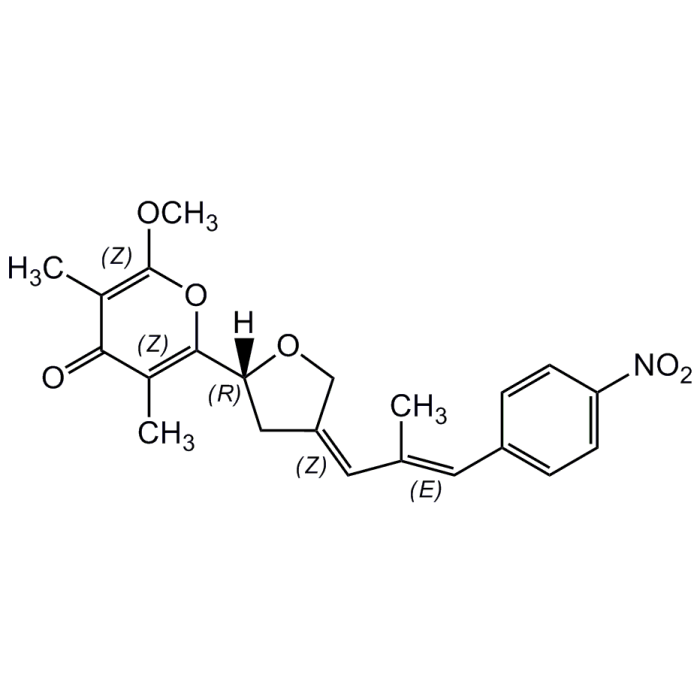
Aureothin
As low as
265
CHF
CHF 265.00
In stock
Only %1 left
BVT-0303-M0011 mgCHF 265.00
BVT-0303-M0055 mgCHF 830.00
| Product Details | |
|---|---|
| Synonyms | Mycolutein; Distacin; JA 2814K; Antibiotic 74A; BRN 0058476 |
| Product Type | Chemical |
| Properties | |
| Formula |
C22H23NO6 |
| MW | 397.4 |
| Merck Index | 10:879 |
| CAS | 2825-00-5 |
| RTECS | UQ0800000 |
| Source/Host Chemicals | Isolated from Streptomyces thioluteus. |
| Purity Chemicals | ≥96% (HPLC) |
| Appearance | Yellow solid. |
| Solubility | Soluble in 100% ethanol, methanol, DMSO, dimethylformamide, dicholormethane or acetone. |
| Identity | Determined by 1H-NMR and UV. |
| Declaration | Manufactured by BioViotica. |
| InChi Key | GQKXCBCSVYJUMI-RCGCBBHSSA-N |
| Smiles | COC1=C(C)C(=O)C(C)=C(O1)C1C\C(CO1)=C\C(\C)=C\C1=CC=C(C=C1)[N+]([O-])=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light when in solution. |
| Use/Stability |
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- NADH dehydrogenase (NADH-Coenzyme Q oxidoreductase; Complex I) inhibitor. Inhibits mitochondrial respiration and oxidative phosphorylation (OXPHOS).
- Antitrypanosomal, antibacterial, antifungal, insecticidal and pesticidal.
- Antitumor compound.
- Potent anti-HIV agent.
Product References
- The structure of aureothin, a nitro compound obtained from Streptomyces thioluteus: Y. Hirata, et al.; Tetrahedron 14, 252 (1961)
- Identification of mycolutein and pulvomycin as aureothin and labilomycin respectively: J.L. Schwartz, et al.; J. Antibiot. 29, 236 (1976)
- Two binding sites of inhibitors in NADH: ubiquinone oxidoreductase (complex I). Relationship of one site with the ubiquinone-binding site of bacterial glucose:ubiquinone oxidoreductase: T. Friedrich, et al.; Eur. J. Biochem. 219, 691 (1994)
- Absolute configuration of (+)-aureothin: A toxic Metabolite posessing gamma-pyrone unit: Y. Ishibashi, et al.; Bull. Chem. Soc. Jpn. 68, 3643 (1995)
- Gamma-pyrone compounds with selective and potent anti-Helicobacter pylori activity: M. Taniguchi, et al.; J. Antibiot. 53, 844 (2000)
- Dissection of the late steps in aureothin biosynthesis: M. Müller, et al.; ChemBioChem 7, 37 (2006)
- New aureothin derivative, alloaureothin, from Streptomyces sp. MM23: J.Y. Ueda, et al.; J. Antibiot. 60, 321 (2007)
- Chemoenzymatic total synthesis of the antiproliferative polyketide (+)-(R)-aureothin: M. Werneburg & C. Hertweck; Chembiochem 9, 2064 (2008)
- Selective and potent in vitro antitrypanosomal activities of ten microbial metabolites: K. Otoguro, et al.; J. Antibiot. 61, 372 (2008)
- Natural lipophilic inhibitors of mitochondrial complex I are candidate toxins for sporadic neurodegenerative tau pathologies: M. Höllerhagen, et al.; Exp. Neurol. 220, 133 (2009)
- Evolution of metabolic diversity in polyketide-derived pyrones: using the non-colinear Aureothin assembly line as a model system: B. Busch, et al.; Phytochemistry 70, 1833 (2009)
- Exploiting enzymatic promiscuity to engineer a focused library of highly selective antifungal and antiproliferative Aureothin analogues: M. Werneburg, et al.; J. Am. Chem. Soc. 132, 10407 (2010)
- Potent inhibition of HIV replication in primary human cells by novel synthetic polyketides inspired by Aureothin: A. Herrmann, et al.; Sci. Rep. 10, 1326 (2020)