Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
BioViotica
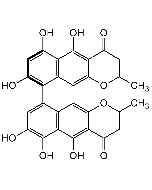
Rugulosin
As low as
110
CHF
CHF 110.00
In stock
Only %1 left
BVT-0444-M0011 mgCHF 110.00
BVT-0444-M0055 mgCHF 395.00

| Product Details | |
|---|---|
| Synonyms | (+)-Rugulosin; NSC 160880; NSC 249990; Radicalisin |
| Product Type | Chemical |
| Properties | |
| Formula |
C30H22O10 |
| MW | 542.5 |
| CAS | 23537-16-8 |
| RTECS | VM1610000 |
| Source/Host Chemicals | Isolated from Penicillium sp. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Yellow solid. |
| Solubility | Soluble in DMSO, acetone, ethanol or methanol. |
| Identity | Determined by 1H-NMR, MS and optical rotation. |
| Declaration | Manufactured by BioViotica. |
| InChi Key | QFDPVUTXKUGISP-UHFFFAOYSA-N |
| Smiles | CC1=CC2=C(C(O)=C1)C(=O)C1=C(O)[C@H]3C(O)C4C5C(O)[C@H](C(O)=C6C(=O)C7=C(O)C=C(C)C=C7C(=O)C356)C14C2=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Keep cool and dry. Protect from light when in solution. |
| Use/Stability | Stable for at least 1 year after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Mycotoxin.
- Antibiotic (Streptococcus, Corynebacterium, MRSA).
- Antiviral, HIV-1 integrase inhibitor.
- DNA replication, transcription and repair inhibitor (carcinogenic activity).
- RNA polymerase and ribonuclease H inhibitor.
- Insecticidal activity.
- Inhibitor of N-Hsp90.
Product References
- Studies in the biochemistry of micro-organisms. 95. Rugulosin, a crystalline colouring matter of Penicillium rugulosum Thom: J. Breen, et al.; Biochem. J. 60, 618 (1955)
- Inhibition of phage growth by an antibiotic rugulosin isolated from Myrothecium verucaria. I. Properties of the anti-phage effect: S. Nakamura, et al.; Jpn. J. Microbiol. 15, 113 (1971)
- Toxicological approach to (+) rugulosin, an anthraquinoid mycotoxin of Penicillium rugulosum Thom: Y. Ueno, et al.; Jpn. J. Exp. Med. 41, 177 (1971)
- Specific and non-specific interactions of two carcinogenic mycotoxins, luteoskyrin and rugulosin with nucleic acids: J.C. Bouhet, et al.; Ann. Nutr. Aliment. 31, 811 (1977)
- Mutagenicity and antibacterial activity of mycotoxins produced by Penicillium islandicum Sopp and Penicillium rugulosum: A.A. Stark, et al.; J. Environ. Pathol. Toxicol. 2, 313 (1978)
- Inhibitory effects of carcinogenic mycotoxins on deoxyribonucleic acid-dependent ribonucleic acid polymerase and ribonuclease H: F. Tashiro, et al.; Appl. Environ. Microbiol. 38, 191 (1979)
- Cytotoxicity against insect cells of entomopathogenic fungi of the genera Hypocrella (anamorph Aschersonia): possible agents for biological control: P. Watts, et al.; Mycol. Res. 107, 581 (2003)
- Isolation, structure, and HIV-1-integrase inhibitory activity of structurally diverse fungal metabolites: S.B. Singh, et al.; J. Ind. Microbiol. Biotechnol. 30, 721 (2003)
- New rugulosins, anti-MRSA antibiotics, produced by Penicillium radicum FKI-3765-2: H. Yamazaki, et al.; Org. Lett. 12, 1572 (2010)
- Inhibition and binding of Rugulosin with N-Hsp90: J.-J.Chen, et al.; Gaodeng Xuexiao Huaxue Xuebao 32, 88 (2011)
- Antibiotically active metabolites from Talaromyces wortmannii, an endophyte of Aloe vera: R. Bara, et al.; J. Antibiot. 66, 491 (2013)
- Distribution of the foliar fungal endophyte Phialocephala scopiformis and its toxin in the crown of a mature white spruce tree as revealed by chemical and qPCR analyses: S. L. Frasz, et al.; Can. J. Forest Res. 44,1138 (2014)
- Comparison of cytotoxic extracts from fruiting bodies, infected insects and cultured mycelia of Cordyceps formosana: R.-L. Lu, et al.; Food Chem. 145, 1066 (2014)