Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
BioViotica
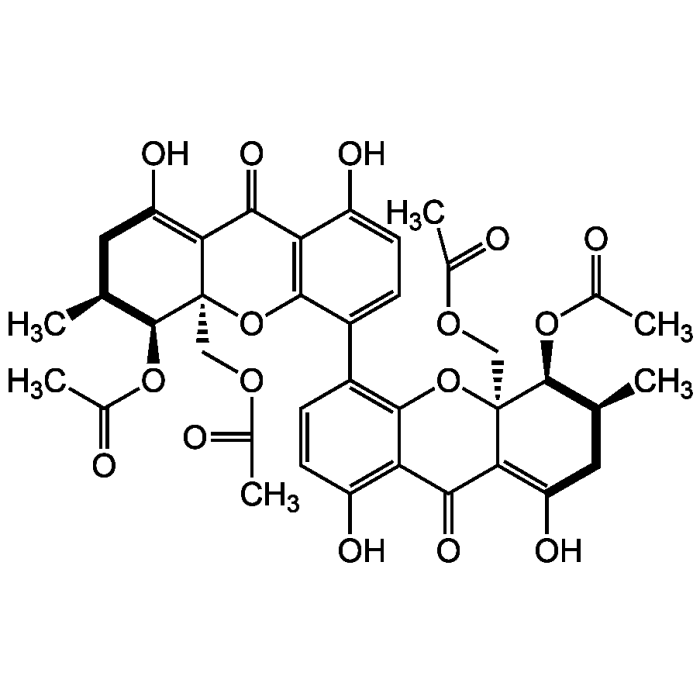
Phomoxanthone A
As low as
385
CHF
CHF 385.00
In stock
Only %1 left
BVT-0453-M0055 mgCHF 385.00
| Product Details | |
|---|---|
| Product Type | Chemical |
| Properties | |
| Formula |
C38H38O16 |
| MW | 750.7 |
| CAS | 359844-69-2 |
| Source/Host Chemicals | Isolated from fungus Phomopsis sp M4. |
| Purity Chemicals | >97% (1H-NMR, HPLC) |
| Appearance | Pale yellow solid. |
| Solubility | Soluble in DMSO, methanol or acetone. Insoluble in water. |
| Identity | Determined by 1H-NMR and MS. |
| Declaration | Manufactured by BioViotica. |
| Other Product Data |
Note: P. Boehler (2018) describe that Phomoxanthone A becomes unstable if dissolved in DMSO, and readily isomerizes into the essentially non-toxic compound dicerandrol C. We advise therefore to dissolve the compound in DMSO immediately before usage. |
| InChi Key | OHHXJWHRQGZQJM-CMDKCIDSSA-N |
| Smiles | C[C@H]1CC(O)=C2C(=O)C3=C(O[C@@]2(COC(C)=O)[C@H]1OC(C)=O)C(=CC=C3O)C1=C2O[C@@]3(COC(C)=O)[C@@H](OC(C)=O)[C@@H](C)CC(O)=C3C(=O)C2=C(O)C=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light when in solution. |
| Use/Stability | Stable for at least 1 year after receipt when stored at +4°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Mycotoxin
- Antibacterial against Gram-positive bacteria.
- Shows antimalarial and antitubercular activity.
- Anticancer compound. Cytotoxic against several cancer cell lines (pro-apoptotic activity).
- Active against plant pathogene fungi (e.g. P. oryza, Phyt. infestans).
- Activator of murine T lymphocytes, NK cells and macrophages.
- Mitochondrial toxin with a mode of action distinct from known electron transport chain (ETC) inhibitors, OXPHOS uncouplers and ionophores. Shows rapid inhibition of both ETC and ΔΨm, the release of mitochondrial Ca2+ and fission of the inner but not the outer mitochondrial membrane independent from the mitochondrial fission and fusion regulators DRP1 and OPA1.
Product References
- Phomoxanthones A and B, novel xanthone dimers from the endophytic fungus Phomopsis species: M. Isaka, et al.; J. Nat. Prod. 64, 1015 (2001)
- X-ray Structure Determination, Absolute Configuration and Biological Activity of Phomoxanthone A: B. Elsasser, et al.; Eur. J. Org. Chem. 2005, 4563 (2005)
- Anticancer compounds derived from fungal endophytes: their importance and future challenges: R.N. Kharwar, et al.; Nat. Prod. Rep. 28, 1208 (2011)
- Isolation of a phomoxanthone A derivative, a new metabolite of tetrahydroxanthone, from a Phomopsis sp. isolated from the mangrove, Rhizhhopora mucronata: Y. Shiono, et al.; Nat. Prod. Commun. 8, 1735 (2013)
- Pro-apoptotic and immunostimulatory tetrahydroxanthone dimers from the endophytic fungus Phomopsis longicolla: D. Rönsberg, et al.; J. Org. Chem. 78, 12409 (2013)
- The mycotoxin phomoxanthone A disturbs the form and function of the inner mitochondrial membrane: P. Boehler, et al.; Cell Death Dis. 9, 286 (2018)






