Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
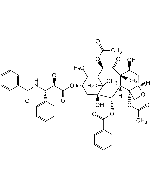
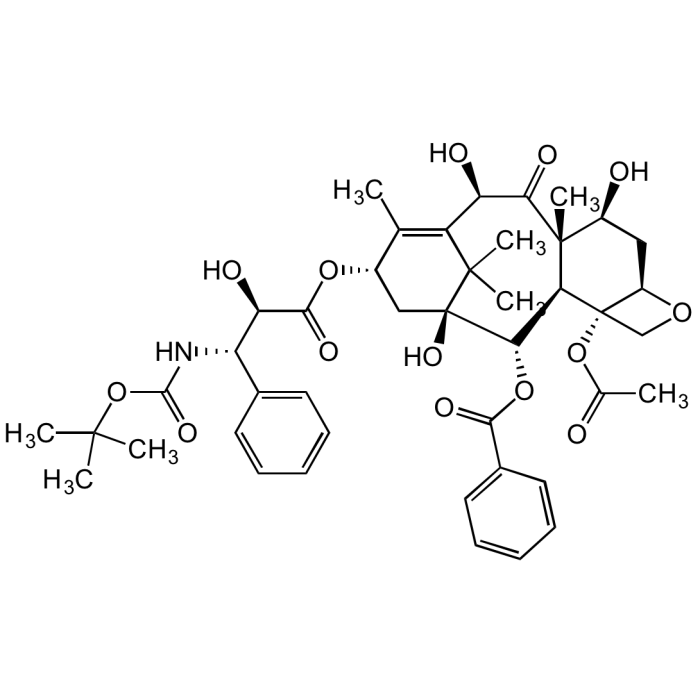
Docetaxel
| Product Details | |
|---|---|
| Synonyms | Taxotere®; NSC 628503; RP 56976; N-debenzoyl-N-tert-butoxycarbonyl-10-deacetyltaxol |
| Product Type | Chemical |
| Properties | |
| Formula |
C43H53NO14 |
| MW | 807.88 |
| CAS | 114977-28-5 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥97% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in DMSO or ethanol. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | ZDZOTLJHXYCWBA-VCVYQWHSSA-N |
| Smiles | CC(C)(OC(N[C@H]([C@H](C(O[C@H]1C[C@@]([C@H]([C@@H]([C@@]2(C([C@@H](C3=C1C)O)=O)C)[C@]4([C@@H](C[C@@H]2O)OC4)OC(C)=O)OC(C5=CC=CC=C5)=O)(C3(C)C)O)=O)O)C6=CC=CC=C6)=O)C |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Anticancer agent. Semisynthetic analog of taxol (paclitaxel). Inhibits microtubule disassembly and inhibits cell replication. Binds to and stabilizes the β−tubulin subunit of microtubules, preventing depolymerization of the mitotic spindle thus leading to cell cycle arrest at G2/M and apoptosis. This cytotoxic activity has been widely exploited in suppressing oncogenic proliferation. Displays potent and broad antineoplastic properties, used mainly for the treatment of breast, ovarian, and non-small cell lung cancer. Also inhibits pro-angiogenic factors such as vascular endothelial growth factor (VEGF) and displays immunomodulatory and pro-inflammatory properties by inducing various mediators of the inflammatory response. Also studied for use as a radiation-sensitizing agent.
(1) M.C. Bissery, et al.; Cancer Res. 51, 4845 (1991) | (2) R. Bruno & G.J. Sanderink; Cancer Surv. 17, 305 (1993) (Review) | (3) K. Gelmon; Lancet 344, 1267 (1994) (Review) | (4) M.E. Trudeau; Can. J. Oncol. 6, 443 (1996) (Review) | (5) I.A. Avramis, et al.; Anticancer Res. 21, 2281 (2001) | (6) M. Aapro & E. Rowinsky; Anticancer Drugs 12, S1 (2001) (Review) | (7) P.B. Cassidy, et al.; Clin. Cancer Res. 8, 846 (2002) | (8) M.S. Si, et al.; Invest. New Drugs 21, 281 (2003) | (9) B. Ramaswamy & S. Puhalla; Drugs Today 42, 265 (2006) (Review) | (10) H. Hernandez-Vargas, et al.; Cell Cycle 6, 780 (2007) (Review) | (11) H. Ching-Hsia, et al.; Toxicol. Sci. 145, 59 (2015)