Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
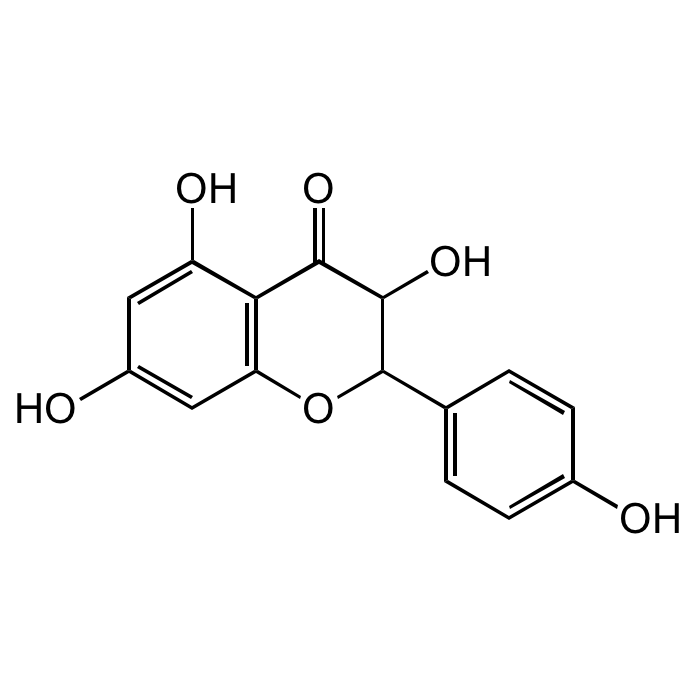
(±)-Dihydrokaempferol
| Product Details | |
|---|---|
| Synonyms | (±)-Aromadendrol; (±)-Aromadendrin; Helicioside A; Katuranin; (2RS,3RS)-3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-2,3-dihydro-4H-chromen-4-one |
| Product Type | Chemical |
| Properties | |
| Formula | C15H12O6 |
| MW | 288.25 |
| CAS | 104486-98-8 |
| Purity Chemicals | ≥95% (HPLC) |
| Appearance | Off-white solid. |
| Solubility | Slightly soluble in water. |
| Identity | Determined by NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | PADQINQHPQKXNL-UHFFFAOYSA-N |
| Smiles | O=C1C(O)C(C2=CC=C(O)C=C2)OC3=C1C(O)=CC(O)=C3 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Keep cool and dry. Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Flavanonol derived from plant source. Precursor of kaempferol. Shown to stimulate glucose uptake and improve insulin resistance by inducing adipogenesis through increased PPAR2 expression. Anti-inflammatory compound.
(1) L. Patschke & H. Grisebach; Phytochemistry 7, 235 (1968)| (2) M.N. Zaprometov & H. Grisebach; Z. Naturforsch. C 28, 113 (1973) | (3) W.Y. Zhang, et al.; Pharmacol. 88, 266 (2011) | (4) J.W. Lee, et al.; Biomol. Ther. 21, 216 (2013)
Aromadendrin: a dual amyloid promoter to accelerate fibrillization and reduce cytotoxicity of both amyloid-b and hIAPP: Y. Zhang, et al.; Royal Soc. Chem. Mat. Sci. 2020