Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
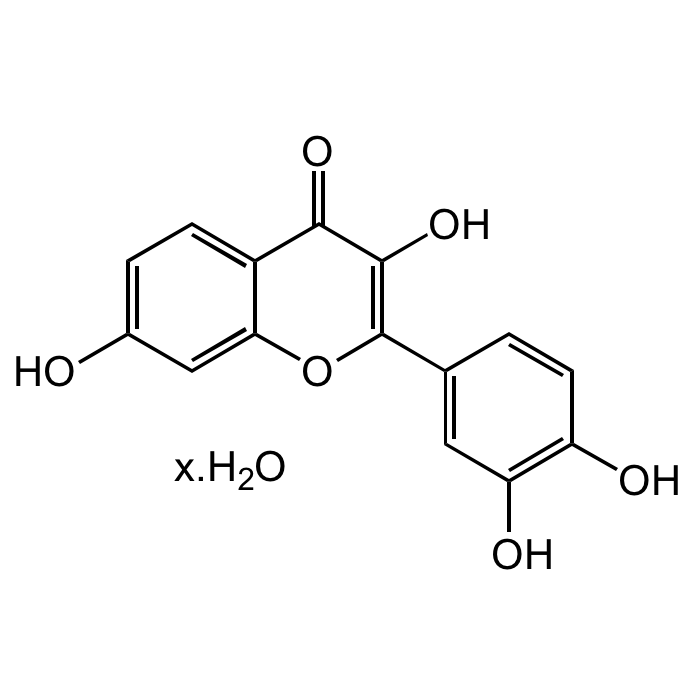
Fisetin
| Product Details | |
|---|---|
| Synonyms | 3,3',4',7-Tetrahydroxyflavone; 5-Deoxyquercetin; Natural Brown 1; CI-75620; NSC 407010; NSC 656275; BRN 0292829; Cotinin |
| Product Type | Chemical |
| Properties | |
| Formula |
C15H10O6 |
| MW | 286.24 |
| CAS | 528-48-3 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Yellow to amber-ochre powder. |
| Solubility | Soluble in DMSO (10mg/ml), DMF (10mg/ml) or ethanol (2mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | XHEFDIBZLJXQHF-UHFFFAOYSA-N |
| Smiles | OC1=CC=C2C(OC(C3=CC(O)=C(O)C=C3)=C(O)C2=O)=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
One of the major polyphenolic flavonoids found in various fruits and vegetables such as apples, grapes, persimmons, strawberries, cucumbers and onions. Exerts numerous beneficial biological activities, including antibacterial, antifungal, antioxidant, anti-inflammatory, antiangiogenic, antiproliferative, proapoptotic, cell cycle arrest, autophagy inducing, hypolipidemic, neuroprotective and antitumor effects. Modulates multiple signaling pathways such as blocking of the phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/Akt/mTOR) and p38, mitogen-activated protein kinases (MAPK)-dependent nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling, Wnt and adrogen receptor signaling, which playing a central role in various cellular processes contributing to the malignancy. Binds to β-tubulin and disrupts microtubule dynamics. Activator of sirtuin 1 and PPARs, inhibitor of DNA topoisomerase II (topo II), inhibitor of CDK6and GSK-3beta. Recently used as fluorescent molecular probe in sodium dodecyl sulfate (SDS) induced Sol-Gel transition.
(1) M. Gabor & E. Eperjessy; Nature 212, 1273 (1966) | (2) A.J. Olaharski, et al.; Mutat. Res. 582, 79 (2005) | (3) B. Sung, et al.; Mol. Pharmacol. 71, 1703 (2007) | (4) N. Khan, et al.; Carcinogenesis 29, 1049 (2008) | (5) B.G. Szczepankiewicz & P.Y. Ng; Curr. Top. Med. Chem. 8, 1533 (2008) (Review) | (6) Y. Suh, et al.; Carcinogenesis 30, 300 (2009) | (7) Y. Suh, et al.; Carcinogenesis 31, 1424 (2010) | (8) R. Singh, et al.; J. Med. Chem. 55, 3614 (2012) | (9) V.M. Adhami, et al.; Biochem. Pharmacol. 84, 1277 (2012) (Review) | (10) D.N. Syed, et al.; Anticancer Agents Med. Chem. 13, 995 (2013) (Review) | (11) N. Khan, et al.; Antioxid. Redox Signal. 19, 151 (2013) (Review) | (12) C.H. Jung, et al.; J. Nutr. Biochem. 24, 1547 (2013) | (13) G.S. Prasath, et al.; Eur. J. Pharmacol. 740, 248 (2014) | (14) T. Jin, et al.; J. Agric. Food Chem. 62, 10468 (2014) | (15) E. Mukhtar, et al.; Cancer Lett. 367, 173 (2015) | (16) M.P. Reis, et al.; J. Appl. Microbiol. 121, 373 (2016) | (17) S.F. Nabavi, et al.; Curr. Top. Med. Chem. 16, 1910 (2016) (Review) | (18) T. Rengarajan & N.S. Yaacob; Eur. J. Pharmacol. 789, 8 (2016) (Review) | (19) M. Youns, et al.; PLoS One 12, e0169335 (2017) | (20) J. Mishra, et al.; J. Phys. Chem. B 122, 181 (2018) | (21) K D. Kashyap, et al.; Life Sci. 194, 75 (2018) (Review) | (22) K. Shanmugam, et al.; Oxid. Med. Cell Longev. 2018, 9173436 (2018)