Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
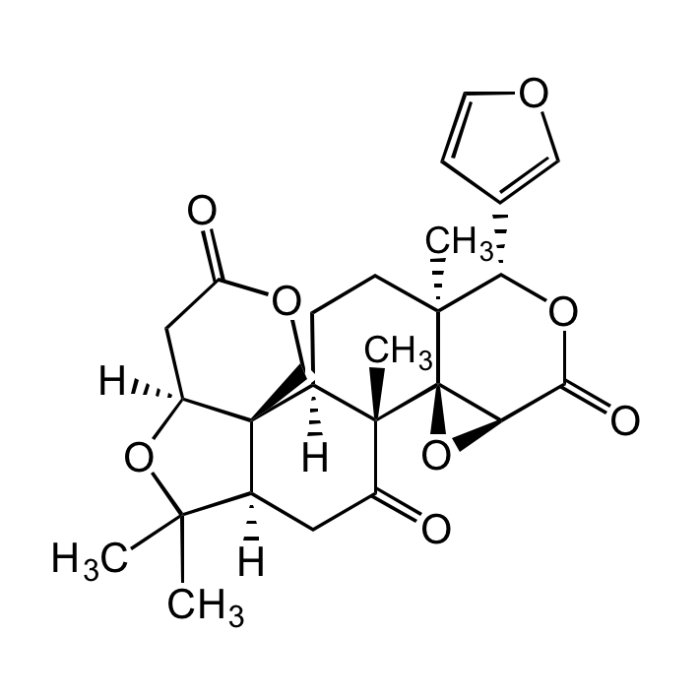
Limonin
| Product Details | |
|---|---|
| Synonyms | 7,16-Dioxo-7,16-dideoxylimondiol; Limonoic acid di-δ-lactone; Citrolimonin; Dictamnolactone; Evodin; Limonine; Obaculactone; NSC 36508 |
| Product Type | Chemical |
| Properties | |
| Formula |
C26H30O8 |
| MW | 470.51 |
| CAS | 1180-71-8 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in DMSO (10mg/ml) or DMF (20mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | KBDSLGBFQAGHBE-MSGMIQHVSA-N |
| Smiles | O=C1[C@@]2(C)[C@@]34O[C@@H]3C(O[C@@H](C5=COC=C5)[C@]4(C)CC[C@]2([H])[C@]67[C@](C1)([H])C(C)(C)O[C@@]6([H])CC(OC7)=O)=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Limonin is the first described limonoid, which are naturally occurring tetranortriterpenoids that impart bitter flavor in citrus fruits. Compounds belonging to this group have reported anti-proliferative and proapoptotic activity on several cancer cell lines as well as pesticidal, anti-malarial, anti-microbial, anti-HIV, anti-inflammatory, antinociceptive and neuroprotective activity. Shown to inhibit arginase I and II and P-glycoprotein. May be used in assays as control, standard or competitor, or in studies on the physiology, metabolism and biosynthesis of limonin.
(1) V.P. Maier, et al.; ACS Symposium Series 143, 63 (1980) | (2) J.A. Klocke & I. Kubo; Ent. Exp. & Appl. 32, 299 (1982) | (3) H. Matsuda, et al.; Planta Med. 64, 339 (1998) | (4) L. Battinelli, et al.; Planta Med. 69, 910 (2003) | (5) A. Roy & S. Saraf; Biol. Pharm. Bull. 29, 191 (2006) (Review) | (6) J.S. Yoon, et al.; J. Nat. Prod. 71, 208 (2008) | (7) J.S. Yoon, et al.; Mol. Neurosci. 42, 9 (2010) | (8) M.Z. El-Readi, et al.; Eur. J. Pharmacol. 626, 139 (2010) | (9) K.N. Chidambara Murthy, et al.; J. Agric. Food Chem. 59, 2314 (2011) | (10) A. Rahman, et al.; Anticancer Agents Med. Chem. 15, 236 (2015) | (11) R. Gualdani, et al.; Molecules 21, E1530 (2016) (Review) | (12) B.H. Koo, et al.; Yonsei Med. J. 59, 366 (2018)