Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
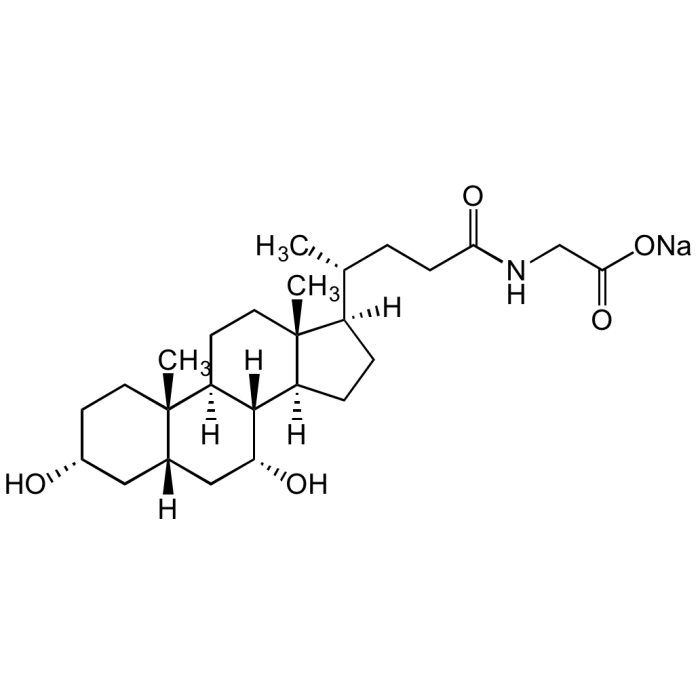
Glycochenodeoxycholic acid . sodium salt
| Product Details | |
|---|---|
| Synonyms | Sodium glycochenodeoxycholate; GCDCA; NSC 681056; Chenylglycine sodium; N-(3α,7α-Dihydroxy-24-oxocholan-24-yl)glycine sodium salt |
| Product Type | Chemical |
| Properties | |
| Formula | C26H42NNaO5 |
| MW | 471.61 |
| CAS | 16564-43-5 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥97% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Slightly soluble in DMSO, ethanol or methanol (10mg/ml) and in water. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | AAYACJGHNRIFCT-YRJJIGPTSA-M |
| Smiles | [H][C@]1([C@@H](CCC(NCC(O[Na])=O)=O)C)CC[C@@]2([H])[C@]3([H])[C@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@@]21C |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +20°C |
| Handling Advice |
Keep cool and dry. Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Glycochenodeoxycholic acid (GCDCA) is a bile salt formed in the liver that acts as a biosurfactant to solubilize lipids for absorption and is itself absorbed. GCDCA functions as a choleretic, increasing the volume of bile secreted from the liver, and a cholagogue, promoting the discharge of bile from the digestive system. GCDCA has been used to demonstrate a role for bile acids in promoting colorectal carcinogenesis and to inhibit calcium hydroxyapatite precipitation to study the pathogenesis of black pigment gallstones. This product has also been shown to induce apoptosis in isolated hepatocytes via modulation of PKC activity. This compound is used as an anionic detergent to solubilize lipids and as a chiral reagent.
(1) S. Barnes, et al.; J. Lipid Res. 20, 952 (1979) | (2) S.M. Qiu, et al.; J. Clin. Invest. 88, 1265 (1991) | (3) B. Gonzalez, et al.; Mol. Cell. Biochem. 207, 19 (2000) | (4) M. Iwata, et al.; Hepatol. Res. 25, 329 (2003) | (5) S. Ishizuka, et al.; Biomed. Res. 33, 159 (2012)