Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
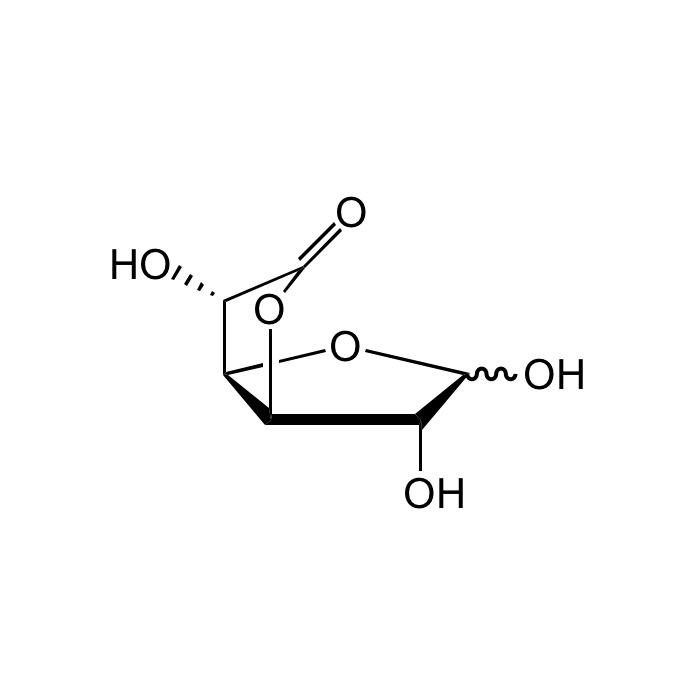
Glucuronolactone
| Product Details | |
|---|---|
| Synonyms | D-(+)-Glucuronic acid γ-lactone; D-(+)-Glucurono-6,3-lactone; D-Glucurono-6,3-lactone; D-Glucurone |
| Product Type | Chemical |
| Properties | |
| Formula | C6H8O6 |
| MW | 176.12 |
| CAS | 32449-92-6 |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White powder. |
| Solubility | Soluble in water. |
| Identity | Determined by NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | OGLCQHRZUSEXNB-VGASXLIASA-N |
| Smiles | O[C@@H]1[C@@H](OC2=O)[C@@H]([C@@H]2O)OC1O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Naturally occurring carbohydrate derivative that is an important structural component of nearly all connective tissues and is also found in many plant gums. Metabolized to glucaric acid, xylitol, and L-xylulose, and humans may also be able to use glucuronolactone as a precursor for ascorbic acid synthesis. Used as a detoxicant. The liver uses glucose to create glucuronolactone, which inhibits the enzyme B-glucuronidase (metabolizes glucuronides), which should cause blood-glucuronide levels to rise. Glucuronides combine with toxic substances, such as morphine and depot medroxyprogesterone acetate, by converting them to water-soluble glucuronide-conjugates which are excreted in the urine. Used as building block and starting reagent for synthesis of drugs, optically active glucopyranoses and long-chain alkyl glucofuranosides.
(1) C.A. Marsh; Biochem. J. 99, 22 (1966) | (2) S.H. Kim, et al.; Acta Crystallogr. 22, 733 (1967) | (3) L. Trahan, et al.; Rev. Can. Biol. 29, 7 (1970) | (4) T. Kuzuya, et al.; Endocrinol. Jpn. 20, 369 (1973) | (5) A. Kirschning, et al.; Bioorg. Med. Chem. Lett. 7, 903 (1997) | (6) S. Suzuki, et al.; J. Chromatogr. Sci. 36, 357 (1998) | (7) A.F. Glawar, et al.; Chemistry 18, 9341 (2012)